Late Permissive Hypercapnia for Mechanically Ventilated Preterm Infants: A Randomized Trial
- PMID: 40525736
- PMCID: PMC12172392
- DOI: 10.1002/ppul.71165
Late Permissive Hypercapnia for Mechanically Ventilated Preterm Infants: A Randomized Trial
Abstract
Objective: To determine if targeting higher levels of pH-controlled permissive hypercapnia beyond postnatal day 7-14 reduces mechanical ventilation duration in preterm infants.
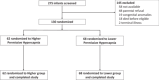
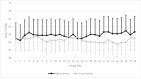
Methods: Single-center randomized clinical trial with a 1:1 parallel allocation including infants from 22-36 weeks' gestation mechanically ventilated for respiratory distress syndrome on postnatal day 7-14. We targeted higher levels of pH-controlled permissive hypercapnia (60-75 mmHg and pH ≥ 7.20) or lower levels of pH-controlled permissive hypercapnia (40-55 mmHg and pH ≥ 7.25) for 28 days after randomization. The primary outcome was the number of days alive and ventilator-free in the 28 days after randomization.
Results: We enrolled 130 infants with a gestational age (mean ± SD) of 24 weeks and 5 days ± 2 weeks and 0 days and birth weight of 657 ± 198 grams from December 2015 to May 2021. Infants randomized to higher levels of pH-controlled permissive hypercapnia had more alive ventilator-free days than infants randomized to lower levels of pH-controlled permissive hypercapnia (11 ± 10 vs. 6 ± 8; p = 0.009). Grade 2-3 bronchopulmonary dysplasia or death before discharge was not significantly lower in the higher carbon dioxide (PCO2) group (30/62 (44%) vs. 45/68 (59%); adjusted odds ratio (aOR) 0.54, 95% confidence intervals (CI) 0.27-1.08; p = 0.08). Grade 2-3 bronchopulmonary dysplasia among survivors at 36 weeks' postmenstrual age did not differ significantly (higher PCO2 19/53 (35%) vs. lower PCO2 28/53 (50%); aOR 0.56, 95% CI 0.27-1.13; p = 0.12).
Conclusions: Targeting higher levels of permissive hypercapnia from postnatal day 7-14 increased the number of days alive and ventilator-free and may be lung protective compared with targeting lower levels.
Trial registration: Clinicaltrials.gov (identifier number NCT02799875). The first infant was enrolled in December 2015 and the trial was not registered until June 2016. The authors confirm that there were no changes made to the Institutional Review Board (IRB) approved trial protocol (dated 10/20/2015) or any amendments made after recruitment started, between the date of first enrollment and the date of clinicaltrials.gov registration, or between study commencement and completion. Furthermore, the authors confirm that the data were not unblinded until after the last infant had been enrolled (March 2021) and discharged from the hospital (August 2021). Study Details | Late Permissive Hypercapnia for Intubated and Ventilated Preterm Infants | ClinicalTrials.gov.
Keywords: artificial; bronchopulmonary dysplasia; hypercapnia; infant; newborn; premature; respiration; respiratory distress syndrome.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Travers is supported by a grant from Owlet Baby Care Inc. for an investigator‐initiated study (clinicaltrials.gov identifier: NCT05774470). The authors report no other relationships or activities that could appear to have influenced the submitted work. The authors have no conflicts of interest relevant to this article to disclose.
Figures
References
-
- Doyle L. W., Andersson S., Bush A., et al., “Expiratory Airflow in Late Adolescence and Early Adulthood in Individuals Born Very Preterm or With Very Low Birthweight Compared With Controls Born at Term or With Normal Birthweight: A Meta‐Analysis of Individual Participant Data,” Lancet Respiratory Medicine 7, no. 8 (2019): 677–686, 10.1016/S2213-2600(18)30530-7. - DOI - PubMed
-
- Islam J. Y., Keller R. L., Aschner J. L., Hartert T. V., and Moore P. E., “Understanding the Short‐ and Long‐Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia,” American Journal of Respiratory and Critical Care Medicine 192, no. 2 (2015): 134–156, 10.1164/rccm.201412-2142PP. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

