HHHFNC Versus NIPPV Delivered by Long Narrow Cannula in Preterm Infants: Randomized Noninferiority Trial
- PMID: 40525751
- PMCID: PMC12172398
- DOI: 10.1002/ppul.71167
HHHFNC Versus NIPPV Delivered by Long Narrow Cannula in Preterm Infants: Randomized Noninferiority Trial
Abstract
Background: Heated humidified high flow (HHHFNC) and nasal intermittent positive pressure ventilation (NIPPV) delivered by cannula with long and narrow tubing (CLNT) are increasingly used in preterm infants for providing noninvasive respiratory support, due to their high comfort level and minimal nasal trauma. Despite their widespread use, no randomized controlled trial has been conducted so far.
Objective: Determine whether HHHFNC is non-inferior to CLNT-NIPPV in providing respiratory support for preterm infants.
Study design: An unblinded, randomized controlled, non-inferiority multicenter trial.
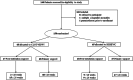
Methodology: Preterm infants randomized to either HHHFNC or CLNT-NIPPV. Infants born > 28 weeks of gestation were eligible to enter the study either as primary treatment after birth or post-extubation. Infants born ≤ 28 weeks of gestation were only eligible post-extubation. The primary outcome was treatment failure within 7 days.
Results: One hundred and thirty infants were enrolled in the study; 65 in each group. Most (82%) were > 28 weeks, and primary treatment (73%). HHHFNC was non-inferior to CLNT-NIPPV in the primary outcome which occurred in 12.3% compared to 23.0% of the infants, respectively (risk difference (RD) -10.77%, 95% CI of RD -23.7 to 2.22 [within the non-inferiority margin], χ2p = 0.168). HHHFNC was associated with significantly less nasal trauma compared to CLNT-NIPPV but with longer time on the allocated respiratory support. No significant differences were found between the groups in secondary respiratory and neonatal outcomes.
Conclusions: In this study of preterm infants mostly > 28 weeks of gestation, HHHFNC was non-inferior to CLNT-NIPPV in providing respiratory support, and caused less nasal trauma.
Keywords: newborn; noninvasive respiratory support; respiratory therapy.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lemyre B., Deguise M. O., Benson P., Kirpalani H., Ekhaguere O. A., and Davis P. G., “Early Nasal Intermittent Positive Pressure Ventilation (NIPPV) Versus Early Nasal Continuous Positive Airway Pressure (NCPAP) for Preterm Infants,” Cochrane Database of Systematic Reviews 7, no. 7 (July 2023): CD005384. - PMC - PubMed
-
- Lemyre B., Deguise M. O., Benson P., Kirpalani H., De Paoli A. G., and Davis P. G., “Nasal Intermittent Positive Pressure Ventilation (NIPPV) Versus Nasal Continuous Positive Airway Pressure (NCPAP) for Preterm Neonates After Extubation,” Cochrane Database of Systematic Reviews 7, no. 7 (July 2023): CD003212. - PMC - PubMed
-
- Ramaswamy V. V., Bandyopadhyay T., Nanda D., et al., “Efficacy of Noninvasive Respiratory Support Modes as Postextubation Respiratory Support in Preterm Neonates: A Systematic Review and Network Meta‐Analysis,” Pediatric Pulmonology 55, no. 11 (November 2020): 2924–2939. - PubMed
-
- Ramaswamy V. V., More K., Roehr C. C., Bandiya P., and Nangia S., “Efficacy of Noninvasive Respiratory Support Modes for Primary Respiratory Support in Preterm Neonates With Respiratory Distress Syndrome: Systematic Review and Network Meta‐Analysis,” Pediatric Pulmonology 55, no. 11 (November 2020): 2940–2963. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

