Color stability of enamel treated with different antioxidant agents following at-home bleaching with 10% hydrogen peroxide
- PMID: 40525811
- PMCID: PMC11900890
- DOI: 10.1590/1678-7757-2024-0056
Color stability of enamel treated with different antioxidant agents following at-home bleaching with 10% hydrogen peroxide
Abstract
Objective: This study evaluated the color stability of enamel submitted to 10% hydrogen peroxide (HP) followed by antioxidants agents, and the pH and antioxidant activity (AA%) of these agents.
Methodology: Bovine enamel-dentin blocks were randomly distributed into groups (n=10/group): GNC (negative control: no treatment); GPC (positive control: bleaching only); TOC_10% (HP+10% α-tocopherol); GT_10% (HP+10% green tea extract); GS_5% (HP+5% grape seed extract); SA_10% (HP+10% sodium ascorbate); QUI_10% (HP+10% quinoa extract); and QC_1% (HP+1% quercetin). Color (ΔE00) and whiteness index (ΔWID) changes were analyzed using a digital spectrophotometer. The pH and AA% were determined using a pH meter and the DPPH method, respectively. Data were analyzed by ANOVA/Tukey's and Dunnett's tests (α=0.05).
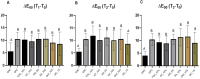
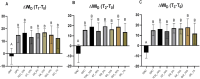
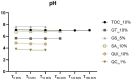
Results: At 14 days post-bleaching, GNC promoted the lowest ΔWID and ΔE00 (p<0.05), and no differences were found between GPC and the remaining groups submitted to the antioxidant agents (p>0.05). QC_1% and QUI_10% exhibited acidic pH levels (3.64 and 4.75, respectively), whereas TOC_10% and GS_5% exhibited alkaline pH (7.07 and 7.64, respectively). No differences in AA% were found between the agents (p>0.05), ranging from 92.6 to 97.6%.
Conclusion: The antioxidant agents did not interfere in bleached enamel color stability, showing satisfactory antioxidant activity. However, QUI and QC gels displayed acidic pH. Clinical significance: The antioxidants evaluated showed high AA% and no impact on post-bleaching color stability, suggesting that their capacity to recover bond strength demonstrated elsewhere would not compromise the esthetic efficacy of tooth bleaching. However, those with acidic pH should be used with caution due to potential enamel damage.
Conflict of interest statement
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Bleaching at acidic and basic pH for enamel and dentin whitening using internal and external techniques.Braz Oral Res. 2025 Jul 7;39:e073. doi: 10.1590/1807-3107bor-2025.vol39.073. eCollection 2025. Braz Oral Res. 2025. PMID: 40638421 Free PMC article.
-
Tropical almond antioxidant gels mitigate the adverse effects of strong whitening agents on immediate bonding.Braz Oral Res. 2025 Jul 7;39:e072. doi: 10.1590/1807-3107bor-2025.vol39.072. eCollection 2025. Braz Oral Res. 2025. PMID: 40638420 Free PMC article.
-
Total sodium replacement by calcium in trimetaphosphate and its incorporation into dental whitening gels: A novel strategy for in-office treatment.J Dent. 2025 Sep;160:105861. doi: 10.1016/j.jdent.2025.105861. Epub 2025 May 31. J Dent. 2025. PMID: 40456382
-
Bleaching effectiveness of hydrogen peroxide containing titanium dioxide: A systematic review and meta-analysis.J Dent. 2025 May;156:105692. doi: 10.1016/j.jdent.2025.105692. Epub 2025 Mar 14. J Dent. 2025. PMID: 40090402
-
Comparison of efficacy of tray-delivered carbamide and hydrogen peroxide for at-home bleaching: a systematic review and meta-analysis.Clin Oral Investig. 2016 Sep;20(7):1419-33. doi: 10.1007/s00784-016-1863-7. Epub 2016 Jun 11. Clin Oral Investig. 2016. PMID: 27290611
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

