Reliability of CMV-IgG kinetics in the diagnosis of CMV primary infection: sensitivity, specificity, and clinical implications
- PMID: 40525841
- PMCID: PMC12323311
- DOI: 10.1128/spectrum.00455-25
Reliability of CMV-IgG kinetics in the diagnosis of CMV primary infection: sensitivity, specificity, and clinical implications
Abstract
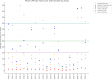
Diagnosis of cytomegalovirus (CMV) primary infection (PI) during pregnancy relies on serology (CMV-IgG, IgM, and IgG avidity). However, as for toxoplasmosis, subsequent serology testing 3-5 weeks later is often performed to confirm the diagnosis. In this study, we aimed to show that testing CMV-IgG with different assays may lead to misinterpretation of CMV-IgG kinetics and to determine the sensitivity and specificity of CMV-IgG stability and significant increase to exclude or confirm recent CMV PI. We conducted a retrospective study on (i) a CMV-IgG external quality control program (2015-2022) and (ii) on CMV serology results obtained in our virology laboratory (2013-2023) in pregnant women with positive CMV-IgM and a subsequent serum sample collected 3-5 weeks later. Analysis of 21 CMV-IgG external quality control serum samples highlighted significant differences in CMV-IgG values, with variations up to a factor of 185 between different immunoassays for the same positive sample. In 434 pregnant women, the sensitivity of a significant CMV-IgG increase to predict recent PI was 32.9% (95% CI = 26.5-39.2), while CMV-IgG stability specificity to exclude PI <3 months was 32.9% (95% CI = 26.5-39.2). Our observations highlight the discrepancies in CMV-IgG values with different assays and the major importance of CMV-IgG avidity in the diagnosis of recent CMV PI in case of positive CMV-IgM. We also demonstrate that retesting IgG on a sample collected 3-5 weeks later is not helpful and can be confusing.IMPORTANCEThis article is the first to address cytomegalovirus (CMV)-IgG kinetics and their reliability in the serological diagnosis of CMV. In our experience, many clinical virologists and laboratory practitioners still rely on kinetics for diagnosis. However, our study clearly demonstrates that this approach is misleading and that avidity testing should always be performed. Additionally, we conducted a robust study highlighting discrepancies between CMV serology techniques, emphasizing the importance for practitioners, particularly gynecologists, to avoid monitoring serology results using different testing methods.
Keywords: CMV; IgG; IgM; avidity; pregnancy; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immune Modulation Related to High-Dose Valacyclovir Administration for Primary Cytomegalovirus Infection in Pregnancy: An Insight into Virus Behavior and Maternal Serology.Viruses. 2025 Jan 24;17(2):157. doi: 10.3390/v17020157. Viruses. 2025. PMID: 40006912 Free PMC article.
-
First-Trimester Universal One-Time Serology Screening for Cytomegalovirus: A Pilot Study at Two Tertiary Referral Centers in Barcelona (Catalunya, Spain).Fetal Diagn Ther. 2025;52(4):388-396. doi: 10.1159/000544169. Epub 2025 Feb 18. Fetal Diagn Ther. 2025. PMID: 39965551 Free PMC article.
-
Congenital cytomegalovirus infection and brain injury in a newborn following maternal non-primary infection: case report of an unexpected diagnosis.Ital J Pediatr. 2025 Jun 21;51(1):197. doi: 10.1186/s13052-025-02017-4. Ital J Pediatr. 2025. PMID: 40542450 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904–1908. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

