Regulatory mechanism for host-cell contact-dependent T3SS gene expression in Vibrio parahaemolyticus
- PMID: 40525855
- PMCID: PMC12282083
- DOI: 10.1128/msystems.00251-25
Regulatory mechanism for host-cell contact-dependent T3SS gene expression in Vibrio parahaemolyticus
Abstract
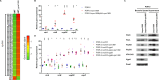
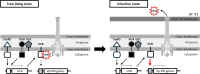
Many pathogenic bacteria regulate gene expression in response to the surrounding environment to establish infection. One such mechanism is the regulation of gene expression in response to contact with host cells. Here, we show that Vibrio parahaemolyticus, a causative agent of foodborne gastroenteritis, has a host-cell contact-dependent regulatory mechanism for virulence gene expression. Type III secretion system 2 (T3SS2), an essential virulence determinant for acute gastroenteritis encoded by V. parahaemolyticus pathogenicity island (Vp-PAI), recognizes host-cell contact by sensing high intracellular K+ levels and switching its secretory substrates. The switching of secretory substrates is regulated by proteins called gatekeepers. Mutants deficient in the gatekeeper genes lose the ability to switch secretory substrates and lock their secretory state into a host-cell contact-dependent manner. Transcriptomic analysis of these mutants revealed the upregulation of Vp-PAI genes, which was dependent on T3SS2 secretory activity, suggesting the presence of a negative regulator secreted by T3SS2. Comparative proteomic analyses identified a previously unrecognized T3SS2 secretory substrate, VPA1369 (VtrN), that negatively regulates Vp-PAI gene transcription. Secretion of VtrN was promoted under conditions that mimic host-cell contact. vtrN gene deletion specifically upregulated Vp-PAI gene expression, independent of T3SS2 secretory activity, indicating its role as a repressor. VtrN interacts with VtrB, a key transcription factor for Vp-PAI genes, suppressing its transcriptional activity. This mechanism illustrates how V. parahaemolyticus enhances virulence gene expression upon host-cell contact through the T3SS2 recognition system, highlighting an adaptive strategy for establishing infection.IMPORTANCEThe type III secretion system (T3SS) is a crucial virulence factor tightly regulated for optimal host manipulation and virulence. This study revealed that the expression of T3SS2, a key virulence factor in Vibrio parahaemolyticus that causes acute gastroenteritis, is strictly regulated by host-cell contact. VtrN, a negative regulator exported from the bacterium through T3SS2, plays a key role in this host-cell contact-dependent gene transcriptional process. VtrN binds directly to the master regulator of Vp-PAI, the region encoding T3SS2, and represses its transcriptional activity. Upon host-cell contact, VtrN export is promoted, leading to the derepression of Vp-PAI gene expression. Thus, V. parahaemolyticus can effectively upregulate the expression of virulence factors when interacting with the host cells. Understanding these regulatory mechanisms could lead to innovative infection control strategies, opening new avenues for research and discovery.
Keywords: Vibrio parahaemolyticus; gatekeeper; gene regulation; type III secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Vibrio parahaemolyticus VtrB contributes to bile resistance and regulatory influences extend beyond the pathogenicity island (Vp-PAI).Microb Pathog. 2025 Sep;206:107818. doi: 10.1016/j.micpath.2025.107818. Epub 2025 Jun 18. Microb Pathog. 2025. PMID: 40541748
-
Role of the TPR family protein VPA1365 in regulating type III secretion system 2 and virulence in Vibrio parahaemolyticus.Appl Environ Microbiol. 2025 Apr 23;91(4):e0220124. doi: 10.1128/aem.02201-24. Epub 2025 Mar 25. Appl Environ Microbiol. 2025. PMID: 40130841 Free PMC article.
-
Virulence Potential of Nonclinical Vibrio parahaemolyticus Isolates From Vietnam: Evidence for Functional T3SS2-Mediated Enterotoxicity.Microbiol Immunol. 2025 Aug;69(8):429-445. doi: 10.1111/1348-0421.13229. Epub 2025 Jun 29. Microbiol Immunol. 2025. PMID: 40582715
-
Advances on Vibrio parahaemolyticus research in the postgenomic era.Microbiol Immunol. 2020 Mar;64(3):167-181. doi: 10.1111/1348-0421.12767. Epub 2020 Jan 21. Microbiol Immunol. 2020. PMID: 31850542 Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- Faculty of Tropical Medicine, Mahidol University
- JP23wm0125006/Japan Agency for Medical Research and Development
- JP223fa627004/Japan Agency for Medical Research and Development
- JP23wm0225023/Japan Agency for Medical Research and Development
- JP23fk0108663/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
