Will gut, oral, and vaginal microbiota influence the outcome of FET or be influenced by FET? A pilot study
- PMID: 40525871
- PMCID: PMC12239586
- DOI: 10.1128/mbio.00509-25
Will gut, oral, and vaginal microbiota influence the outcome of FET or be influenced by FET? A pilot study
Erratum in
-
Correction for Zhang et al., "Will gut, oral, and vaginal microbiota influence the outcome of FET or be influenced by FET? A pilot study".mBio. 2025 Sep 10;16(9):e0231325. doi: 10.1128/mbio.02313-25. Epub 2025 Aug 11. mBio. 2025. PMID: 40788060 Free PMC article. No abstract available.
Abstract
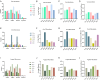
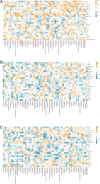
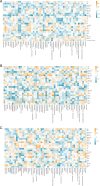
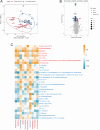
This study aims to examine the specific relationships between gut, oral, and vaginal microbiota and the frozen embryo transfer (FET) process. Patients undergoing fertility treatment who met the inclusion criteria were included in this study. After sampling at three time points, participants were then divided into two groups: the failure group and the success group, based on whether a viable intrauterine pregnancy was confirmed. In this pilot study, we systematically examined changes in the gut, oral, and vaginal microbiota at various stages of the FET process using 16S rDNA high-throughput sequencing and investigated their respective associations with FET outcomes. Metabolomics and random forest were used for evaluating the relationship between gut microbiota and metabolites during FET. Our findings indicate that while the gut microbiota underwent the least change throughout FET, it exhibited the greatest differences between success and failure groups. The oral and vaginal microbiota exhibited significant fluctuations. However, the differences in oral microbiota between the success and failure groups changed with the FET process, while the vaginal microbiota did not show any differences. Notably, two key gut genera, Anaerococcus and Negativicoccus, were identified as genera significantly associated with FET outcomes. Additionally, specific gut microbiota and metabolite profiles displayed significant correlations with FET success, particularly highlighting the potential relevance of cystamine before FET. These findings suggest that targeting microbiota-associated metabolic pathways may serve as a potential strategy to enhance FET success rates and provide new biomarkers for clinical prediction and intervention.IMPORTANCEThis study explores the potential role of microbiota in influencing FET outcomes. Through an analysis of gut, oral, and vaginal microbiota, we observed notable differences between success and failure groups, particularly in gut microbiota. Genera such as Anaerococcus and Negativicoccus, along with associated metabolic profiles, may offer insights into underlying mechanisms. These findings contribute to a growing understanding of the interplay between microbiota and reproductive outcomes and suggest that targeting microbiota-associated metabolic pathways could be a promising direction for enhancing FET success rates. This research highlights potential biomarkers and therapeutic avenues for further exploration in fertility treatments.
Keywords: frozen embryo transfer; gut microbiota; metabolism; oral microbiota; vaginal microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
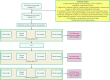
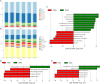

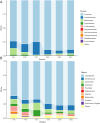

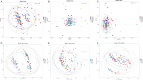



References
-
- Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, Holmes E, Nicholson JK, Teoh TG, MacIntyre DA. 2017. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 5:6. doi: 10.1186/s40168-016-0223-9 - DOI - PMC - PubMed
-
- Zhao C, Wei Z, Yang J, Zhang J, Yu C, Yang A, Zhang M, Zhang L, Wang Y, Mu X, Heng X, Yang H, Gai Z, Wang X, Zhang L. 2020. Characterization of the vaginal microbiome in women with infertility and its potential correlation with hormone stimulation during in vitro fertilization surgery. mSystems 5:e00450-20. doi: 10.1128/mSystems.00450-20 - DOI - PMC - PubMed
-
- Carosso A, Revelli A, Gennarelli G, Canosa S, Cosma S, Borella F, Tancredi A, Paschero C, Boatti L, Zanotto E, Sidoti F, Bottino P, Costa C, Cavallo R, Benedetto C. 2020. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: a pilot study. J Assist Reprod Genet 37:2315–2326. doi: 10.1007/s10815-020-01878-4 - DOI - PMC - PubMed
-
- Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD, Poort L, Cuypers WJSS, Beckers NGM, Broekmans FJM, Cohlen BJ, den Hartog JE, Fleischer K, Lambalk CB, Smeenk JMJS, Budding AE, Laven JSE. 2019. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 34:1042–1054. doi: 10.1093/humrep/dez065 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

