Novel C5α-substituted carbapenems enhance Mycobacterium abscessus killing via selective target binding and reduced hydrolysis by BlaMab
- PMID: 40526087
- PMCID: PMC12326981
- DOI: 10.1128/aac.00170-25
Novel C5α-substituted carbapenems enhance Mycobacterium abscessus killing via selective target binding and reduced hydrolysis by BlaMab
Abstract
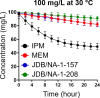
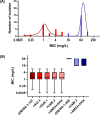
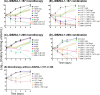
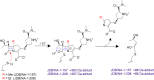
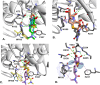
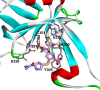
Mycobacterium abscessus (Mab) poses significant clinical challenges and underscores the urgent need for safer and more effective treatments, including β-lactams. Among currently available carbapenems, imipenem is widely used to treat Mab infections by combining with other antibiotics. Commercial carbapenems share a common scaffold with C2 modifications, whereas this study focuses on novel carbapenem candidates with C5α modifications. We evaluated their antibacterial activity against Mab ATCC 19977 and clinical isolates, as well as their acylation of peptidoglycan target receptors (L,D-transpeptidases [LDTs] and penicillin-binding proteins [PBPs]) and the β-lactamase enzyme BlaMab. In vitro studies of two C5α-modified carbapenems, JDB/NA-1-157 and JDB/NA-1-208, revealed distinct antibacterial effects. JDB/NA-1-157 demonstrated potent bacterial killing with low minimum inhibitory concentrations (MICs; 0.125-8 mg/L) and near-complete eradication within 5 days, surpassing the efficacy of the standard-of-care regimen (amikacin + clarithromycin + imipenem). In contrast, JDB/NA-1-208 exhibited poor bacterial killing, with high MICs (16-256 mg/L) and limited efficacy in time-kill studies. However, JDB/NA-1-208 showed synergistic killing when combined with other β-lactams. Mechanistically, JDB/NA-1-208 is not a substrate for BlaMab, while JDB/NA-1-157 is, albeit with low catalytic efficiency. This is supported by the observation that the addition of avibactam did not enhance synergy with JDB/NA-1-157. The substantial bacterial killing effect of JDB/NA-1-157 is attributed to its high binding affinity for PBP-B, PBP-lipo, PonA2, D,D-carboxypeptidase, and LDT1-2. These findings highlight the potential of novel C5α-modified carbapenems, particularly JDB/NA-1-157, as promising therapeutic candidates for Mab infections.
Keywords: L,D-transpeptidases; Mycobacterium abscessus; carbapenems; penicillin-binding proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
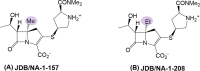


Similar articles
-
Durlobactam in combination with β-lactams to combat Mycobacterium abscessus.Antimicrob Agents Chemother. 2025 Feb 13;69(2):e0117424. doi: 10.1128/aac.01174-24. Epub 2024 Dec 23. Antimicrob Agents Chemother. 2025. PMID: 39714147 Free PMC article.
-
Atypically Modified Carbapenem Antibiotics Display Improved Antimycobacterial Activity in the Absence of β-Lactamase Inhibitors.ACS Infect Dis. 2021 Aug 13;7(8):2425-2436. doi: 10.1021/acsinfecdis.1c00185. Epub 2021 Jun 30. ACS Infect Dis. 2021. PMID: 34191496 Free PMC article.
-
Exploring β-lactam interactions with DacB1: unraveling optimal therapies for combating drug-resistant Mycobacterium tuberculosis.mBio. 2025 Aug 13;16(8):e0137225. doi: 10.1128/mbio.01372-25. Epub 2025 Jul 10. mBio. 2025. PMID: 40637416 Free PMC article.
-
The In vitro activity of carbapenems alone and in combination with β-lactamase inhibitors against difficult-to-treat mycobacteria; Mycobacterium tuberculosis, Mycobacterium abscessus, and Mycobacterium avium complex: A systematic review.Int J Mycobacteriol. 2023 Jul-Sep;12(3):211-225. doi: 10.4103/ijmy.ijmy_131_23. Int J Mycobacteriol. 2023. PMID: 37721224
-
Decoding the Penicillin-Binding Proteins with Activity-Based Probes.Acc Chem Res. 2025 Jun 3;58(11):1754-1763. doi: 10.1021/acs.accounts.5c00113. Epub 2025 May 21. Acc Chem Res. 2025. PMID: 40396497 Review.
References
-
- Jhun BW, Moon SM, Jeon K, Kwon OJ, Yoo H, Carriere KC, Huh HJ, Lee NY, Shin SJ, Daley CL, Koh WJ. 2020. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 55:55. doi: 10.1183/13993003.00798-2019 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

