Multicenter clinical trial for the treatment of obstructive sleep apnea with a non-permanent orthodontic intraoral device in children
- PMID: 40526156
- PMCID: PMC12174215
- DOI: 10.1007/s00431-025-06254-x
Multicenter clinical trial for the treatment of obstructive sleep apnea with a non-permanent orthodontic intraoral device in children
Abstract
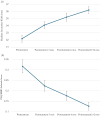
The purpose of this study is to evaluate the safety and efficacy of a non-permanent orthodontic oral appliance intermittently worn in reducing the signs and symptoms of pediatric obstructive sleep apnea (OSA). A non-randomized interventional pre-post study was conducted in 2018-2021 with up to 24-month follow-up at five US and Canadian sites. Participants were 55 enrolled OSA-diagnosed children, fit with a customized device worn in the evening and while sleeping to provide slow maxillary expansion. Participants were assessed for treatment efficacy by three co-primary endpoints, the Pediatric Sleep Questionnaire (PSQ), apnea-hypopnea index (AHI), and intermolar width; and two secondary endpoints, the PSQ Sleep-Related Breathing Disorders (SRBD) subscale scores and airway volume by cone-beam computed tomography (CBCT) scans. Forty-seven participants were included in the analytic dataset following trial completion after 12-24 months. PSQ symptom scores decreased 31.0% with a posttreatment-pretreatment difference score mean±SE and 95% CI of -0.13 ± 0.019 [-0.17, -0.09]; AHI decreased 29.3% (-3.47±1.015 [-5.52, -1.42]). All participants showed an intermolar width increase, averaging 13.0% (4.03 ± 0.421 [3.18, 4.88]). PSQ SRBD subscale scores decreased 57.8% (-0.178 ± 0.031 [-0.24, -0.12]). Airway volume by CBCT scans increased 67.8% (4053.78 ± 885.433 mm3 [2265.62, 5841.95]). There were no safety concerns.
Conclusion: Participants showed objective and subjective OSA improvement, and all demonstrated maxillary expansion. Seventy-nine percent of participants showed AHI improvement, with 61.7% improving by 50% or more, and 17% resolved their OSA. Seventy-seven percent with moderate or severe OSA improved by 50%, while 93% with severe OSA achieved this milestone. This is the first study demonstrating that slow maxillary expansion by this device is safe and efficacious in treating children with OSA.
Trial registration: ClinicalTrials.gov ID NCT05661747 ( https://clinicaltrials.gov/ct2/show/NCT05661747 ) Registered: December 14, 2022, retrospectively registered.
What is known: • Adenotonsillectomy is the primary treatment for pediatric obstructive sleep apnea (OSA) but is invasive and has proximal and long-term risks. Secondary treatments include rapid maxillary expansion that has numerous adverse effects due to its 24/7 use and treatment speed, and positive airway pressure that is limited by patient intolerance and nonadherence.
What is new: • This oral appliance is the first one FDA cleared for OSA treatment in children, and this study showed that slow maxillary expansion by this device was safe and efficacious for treating pediatric OSA. It is also the only pediatric non-permanent orthodontic device that is removable and intermittently worn to treat OSA.
Keywords: Oral appliance; Orthodontic device; Pediatric obstructive sleep apnea.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. The protocol was approved by the WCG Institutional Review Board. Consent to participate: Written informed consent was obtained from all legally authorized representatives of the individual participants included in the study. Consent for publication: Not applicable. Competing interests: The clinical sites received no funding from the sponsor (Vivos Therapeutics, Inc.). Author Dr. Clete Kushida receives fees and stock options for his role as chair of the sponsor’s medical advisory board. Author Dr. Michael Bennett receives fees from the sponsor for his mentorship of dentists who receive sponsor training in the oral appliance treatment. Author Dr. Tammarie Heit receives honoraria and travel expenses from the sponsor for educational activities. Authors Jesse Cozean and Dr. Colette Cozean were paid by The EyeDeas Company, which served as the regulatory consultant for the sponsor for its services in IRB/clinicaltrials.gov communication, site training, collecting/preliminarily analyzing data, and writing of the manuscript.
Figures
Similar articles
-
Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children.Cochrane Database Syst Rev. 2015 Oct 14;2015(10):CD011165. doi: 10.1002/14651858.CD011165.pub2. Cochrane Database Syst Rev. 2015. PMID: 26465274 Free PMC article.
-
Drug therapy for obstructive sleep apnoea in adults.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD003002. doi: 10.1002/14651858.CD003002.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2013 May 31;(5):CD003002. doi: 10.1002/14651858.CD003002.pub3. PMID: 16625567 Updated.
-
Orthodontic treatment for prominent lower front teeth (Class III malocclusion) in children.Cochrane Database Syst Rev. 2024 Apr 10;4(4):CD003451. doi: 10.1002/14651858.CD003451.pub3. Cochrane Database Syst Rev. 2024. PMID: 38597341 Free PMC article.
-
Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea.Cochrane Database Syst Rev. 2015 Jul 14;(7):CD011090. doi: 10.1002/14651858.CD011090.pub2. Cochrane Database Syst Rev. 2015. PMID: 26171909
-
A randomized controlled trial comparing treatment efficacy between rapid maxillary expansion and adenotonsillectomy in pediatric obstructive sleep apnea.Sleep Breath. 2025 Jul 30;29(4):256. doi: 10.1007/s11325-025-03427-8. Sleep Breath. 2025. PMID: 40739069 Free PMC article. Clinical Trial.
References
-
- Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J et al (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 130(3):e714-55. 10.1542/peds.2012-1672 - PubMed
-
- Marcus CL, Ward SL, Mallory GB, Rosen CL, Beckerman RC, Weese-Mayer DE et al (1995) Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr. 127(1):88–94. 10.1016/s0022-3476(95)70262-8 - PubMed
-
- Marcus CL, Rosen G, Ward SL, Halbower AC, Sterni L, Lutz J et al (2006) Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 117(3):e442-51. 10.1542/peds.2005-1634 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical