Cytomegalovirus reactivation in patients with large B-cell lymphoma treated with chimeric antigen receptor T-cell therapy
- PMID: 40526215
- PMCID: PMC12572091
- DOI: 10.1007/s12185-025-04023-y
Cytomegalovirus reactivation in patients with large B-cell lymphoma treated with chimeric antigen receptor T-cell therapy
Abstract
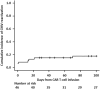
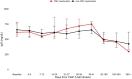
Chimeric antigen receptor (CAR) T-cell therapy has improved outcomes of relapsed and/or refractory large B-cell lymphoma (r/r LBCL). However, its off-tumor effects result in severe prolonged humoral immune deficiency. Cytomegalovirus (CMV) is a latent virus that can be life-threatening in immunosuppressed patients. In the setting of CAR T-cell therapy, Asian race is a risk factor for clinically significant CMV infection. However, the effect of CAR T-cell therapy on CMV reactivation in Japanese patients remains unclear. Previous reports used polymerase chain reaction (PCR), but we used the pp65 antigenemia assay to retrospectively investigate long-term effects in patients with r/r LBCL. The study included 46 patients. Nine (19.6%) developed CMV reactivation, with a median onset of 13 days. Six of these patients received preemptive therapy, and none developed CMV end-organ disease. Primary refractory disease, grade 2-4 cytokine release syndrome, and high-dose corticosteroids were risk factors for CMV reactivation. Long-term follow-up showed that CMV reactivation rarely occurred later than 28 days post-infusion. Our study using the pp65 antigenemia assay showed a similar incidence of CMV reactivation, onset, and risk factors to those in the previous reports using PCR.
Keywords: CAR T-cell therapy; Cytomegalovirus reactivation; Hypogammaglobulinemia; Large B-cell lymphoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: T.M. has received honoraria from Bristol Myers Squibb. D.E., N.F., and Y.M. have received honoraria from Novartis, Bristol Myers Squibb, Gilead Sciences, and Janssen Pharmaceutical. K.F. has received honoraria from Bristol Myers Squibb and Gilead Sciences. The others have no conflict of interest for this study. Ethics approval: All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review board (#2405-051). Consent to participate: This is a retrospective observational study and obtaining written consent was not mandatory. All participants did not provide any denial to this study documents that were made available to them on an opt-out manner on the Web. The opt-out method was approved by the institutional review board.
Figures

References
-
- Bupha-Intr O, Haeusler G, Chee L, Thursky K, Slavin M, Teh B. CAR-T cell therapy and infection: a review. Expert Rev Anti Infect Ther. 2021;19:749–58. - PubMed
-
- Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67:533–40. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

