Long-Term Mortality and Morbidity Impact on Patients with Pulmonary Arterial Hypertension (PAH) If Access to Sotatercept Is Delayed: A Simulation Model
- PMID: 40526255
- PMCID: PMC12313736
- DOI: 10.1007/s12325-025-03241-4
Long-Term Mortality and Morbidity Impact on Patients with Pulmonary Arterial Hypertension (PAH) If Access to Sotatercept Is Delayed: A Simulation Model
Abstract
Introduction: Pulmonary arterial hypertension (PAH) is a rare, progressive disease associated with significant morbidity and mortality. The phase 3 STELLAR trial tested sotatercept plus background therapy (BGT) versus placebo plus BGT, where BGT included mono-, double-, or triple-PAH targeted therapy. Building on the trial's findings, a population health model was recently published assessing the long-term clinical impact of sotatercept. This analysis expands on this model and compares the clinical outcomes of immediate treatment initiation with sotatercept plus BGT against delayed treatment initiation with sotatercept plus BGT using a six-state Markov-type model and over a lifetime horizon.
Methods: State-transition probabilities were obtained from STELLAR, while mortality rates adjusted for risk strata and probabilities of lung/heart-lung transplants were derived from COMPERA PAH registry data and literature.
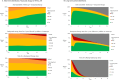
Results: In the base case, a 2-year delay in treatment with sotatercept plus BGT resulted in an average of 12.4 years life expectancy, whereas immediate initiation of sotatercept led to an average of 16.5 years, a difference of 4.1 years. Immediate treatment with sotatercept plus BGT was also associated with a gain in infused prostacyclin-free life-years and resulted in 210 PAH hospitalizations avoided and 5 lung/heart-lung transplant avoided per 1000 patients.
Conclusions: This research suggests that early addition of sotatercept to BGT has the potential to increase life expectancy among patients with PAH and to reduce PAH hospitalizations, prostacyclin-use, and lung/heart-lung transplants needs. Real-world data are needed to confirm these findings, guiding clinicians and healthcare decision-makers in optimizing PAH treatment strategies.
Trial registration: ClinicalTrials.gov identifier, NCT04576988 (STELLAR).
Keywords: Pulmonary arterial hypertension; Risk strata; STELLAR; Sotatercept; Treatment delay.
© 2025. Merck & Co., Inc., Rahway, NJ, USA and its affiliates, and Vallerie McLaughlin, Christine Pausch, Marius M. Hoeper.
Conflict of interest statement
Declarations. Conflict of Interest: Wenjie Zhang and Gijs van de Wetering are employees of OPEN Health, which received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct the study. Adnan Alsumali, Janethe de Oliveira Pena, Rogier Klok, Dominik Lautsch, Jestinah Chevure, Murvin Jootun and Eliana Martinez are employees or were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, at the time the study was done and may hold stock in Merck & Co., Inc., Rahway, NJ, USA. Vallerie McLaughlin is a consultant for Aerovate, Altavant Sciences, Bayer, CVS Caremark, CorVista Health, Gossamer Bio, Janssen, Merck, and United Therapeutics; has received grants from Aerovate, Altavant Sciences, Merck, Gossamer Bio, Janssen, and SoniVie; and received stock from Clene. Christine Pausch has nothing to disclose. Marius M. Hoeper is a consultant and speaker for Acceleron Pharma, Inc., Actelion, AOP Orphan Pharmaceuticals, Bayer, Ferrer, GlaxoSmithKline, Janssen, and MSD. Ethical Approval: This study relied on previously conducted studies and did not involve any new studies requiring the direct participation of human subjects; thus, ethics board approval was not required. The authors sought and received permission from the registry owners to access and use data from the COMPERA PAH registry.
Figures
Similar articles
-
Population Health Model Predicting the Long-Term Impact of Sotatercept on Morbidity and Mortality in Patients with Pulmonary Arterial Hypertension (PAH).Adv Ther. 2024 Jan;41(1):130-151. doi: 10.1007/s12325-023-02684-x. Epub 2023 Oct 18. Adv Ther. 2024. PMID: 37851297 Free PMC article.
-
Pericardial Effusion and Prostacyclin Analog Toxicity After Initiation of Sotatercept.Pulm Circ. 2025 Jul 27;15(3):e70141. doi: 10.1002/pul2.70141. eCollection 2025 Jul. Pulm Circ. 2025. PMID: 40717711 Free PMC article.
-
Macitentan and Tadalafil Combination Therapy in Patients with Pulmonary Arterial Hypertension and Cardiovascular Comorbidities: Real-World Evidence from OPUS and OrPHeUS.Adv Ther. 2025 Jul;42(7):3306-3333. doi: 10.1007/s12325-025-03180-0. Epub 2025 May 19. Adv Ther. 2025. PMID: 40388087 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2006 Sep;10(31):iii-iv, xiii-xvi, 1-239. doi: 10.3310/hta10310. Health Technol Assess. 2006. PMID: 16948890
References
-
- McLaughlin VV, Alsumali A, Lui R, Martinez EC, Nourhussein I, Klok R, et al. Impact of Delaying Access to Sotatercept on Patients Living With Pulmonary Arterial Hypertension. C37 HEALTH SERVICES RESEARCH IN PULMONARY HYPERTENSION. American Thoracic Society International Conference Abstracts: American Thoracic Society; 2024. p. A5429-A.
-
- Kirson NY, Birnbaum HG, Ivanova JI, Waldman T, Joish V, Williamson T. Prevalence of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension in the United States. Curr Med Res Opin. 2011;27(9):1763–8. - PubMed
-
- Ruopp NF, Cockrill BA. Diagnosis and treatment of pulmonary arterial hypertension: a review. JAMA. 2022;327(14):1379–91. - PubMed
-
- Burgoyne DS. Reducing economic burden and improving quality of life in pulmonary arterial hypertension. Am J Manag Care. 2021;27(3 Suppl):S53–8. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

