Role of Nuclear Receptors on the Progression of Multiple Sclerosis: A Review
- PMID: 40526306
- PMCID: PMC12174042
- DOI: 10.1007/s10571-025-01563-z
Role of Nuclear Receptors on the Progression of Multiple Sclerosis: A Review
Abstract
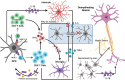
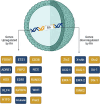
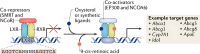
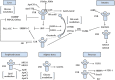
Multiple sclerosis (MS) is the consequence of early-onset inflammatory demyelination and progressive neurodegenerative lesions in the central nervous system (CNS). Despite the unclear pathogenesis of MS, two main processes occur, oligodendrocyte repair and lipid metabolism. Nuclear receptors have been observed to have a role as ligand sensors, regulators of transcriptional factors, and modulators of gene expression. One member of this family, the retinoid X receptor (RXR), which forms a heterodimeric complex with liver X receptor (LXR), have been implicated in the pathophysiology of MS. Moreover, it has been expressed that LXR receptors play pivotal role in the regulation of inflammation, oxidative stress responses, and cholesterol metabolism in phagocytes in active MS lesions. This review aimed to study role of RXR and LXR in MS pathogenesis.
Keywords: Inflammation; LXR; Multiple sclerosis; Neurodegeneration; RXR.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests. Ethical Approval: No ethic approval required.
Figures

Similar articles
-
The Inhibition Mechanism of Pancreatic Ductal Adenocarcinoma via LXR Receptors: A Multifaceted Approach Integrating Molecular Docking, Molecular Dynamics and Post-MD Inter-Molecular Contact Analysis.Asian Pac J Cancer Prev. 2023 Dec 1;24(12):4103-4109. doi: 10.31557/APJCP.2023.24.12.4103. Asian Pac J Cancer Prev. 2023. PMID: 38156844 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Liver X receptors: A therapeutic target in demyelinating disorders.Pharmacol Res. 2025 Sep;219:107861. doi: 10.1016/j.phrs.2025.107861. Epub 2025 Jul 20. Pharmacol Res. 2025. PMID: 40695410 Review.
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Self-talk in a Patient With Down Syndrome: When Is It a Concern?J Dev Behav Pediatr. 2025 Apr 2;46(3):e326-e328. doi: 10.1097/DBP.0000000000001359. J Dev Behav Pediatr. 2025. PMID: 40193695
References
-
- Altucci L et al (2007) RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6(10):793–810 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

