RRP9 promotes prostate cancer metastasis and epithelial-mesenchymal transition through activation of the AKT/GSK3β/β-Catenin signaling pathway
- PMID: 40526312
- PMCID: PMC12173975
- DOI: 10.1007/s12672-025-02833-4
RRP9 promotes prostate cancer metastasis and epithelial-mesenchymal transition through activation of the AKT/GSK3β/β-Catenin signaling pathway
Abstract
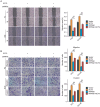
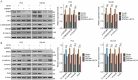
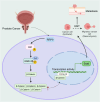
Ribosomal RNA Processing 9 (RRP9) is a gene associated with ribosomal function, and studies have demonstrated that its expression is aberrantly regulated in various tumor types, correlating with tumor progression. However, the specific role and underlying mechanism of RRP9 in prostate cancer (PCa) remain largely unexplored. In this study, bioinformatics analysis revealed that RRP9 is upregulated in PCa and is significantly associated with poor prognosis and lymph node metastasis. Further experimental data demonstrated that RRP9 knockdown notably inhibited the metastasis, invasion, and epithelial-mesenchymal transition (EMT) of PCa. Conversely, overexpression of RRP9 activated the AKT signaling pathway, resulting in the phosphorylation of GSK3β at Ser9, which in turn prevented β-catenin degradation and promoted cell metastasis, invasion, and EMT. Rescue experiments demonstrated that SC79 effectively reversed the inhibitory effects of RRP9 knockdown on PCa. These findings highlight the potential role of RRP9 in promoting the malignant biological behaviors of PCa, providing new insights and potential therapeutic strategies for the treatment of the disease.
Keywords: AKT; Epithelial-mesenchymal transition; GSK3β; Metastasis; Prostate cancer; RRP9; β-catenin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Inhibition of the PI3K/AKT signaling pathway blocks the oncogenic activity of TRIM26 in prostate cancer cells.Asian J Androl. 2025 Jul 8. doi: 10.4103/aja202526. Online ahead of print. Asian J Androl. 2025. PMID: 40625052
-
Targeting Drp1 inhibits ESCC progression via the ROS-PGC1-α-Nrf1/2 pathway.J Transl Med. 2025 Jun 17;23(1):674. doi: 10.1186/s12967-025-06697-8. J Transl Med. 2025. PMID: 40528181 Free PMC article.
-
2'‑Hydroxyflavanone inhibits epithelial‑mesenchymal transition, and cell migration and invasion via suppression of the Wnt/β‑catenin signaling pathway in prostate cancer.Oncol Rep. 2018 Nov;40(5):2836-2843. doi: 10.3892/or.2018.6678. Epub 2018 Aug 31. Oncol Rep. 2018. PMID: 30226607
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Olaparib Monotherapy or in Combination with Abiraterone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and a BRCA Mutation.Target Oncol. 2025 May;20(3):445-466. doi: 10.1007/s11523-025-01146-4. Epub 2025 May 21. Target Oncol. 2025. PMID: 40397306 Free PMC article. Review.
References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Matsukawa A, Yanagisawa T, Bekku K, et al. Nonsurgical interventions to prevent disease progression in prostate cancer patients on active surveillance: a systematic review and meta-analysis. Eur Urol Oncol. 2024;7(3):376–400. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Freitas PFS, Abdshah A, McKay RR, et al. HSD3B1, prostate cancer mortality and modifiable outcomes. Nat Rev Urol. 2024. 10.1038/s41585-024-00953-0. - PubMed
LinkOut - more resources
Full Text Sources
