Erenumab for Chronic Cluster Headache: A Randomized Clinical Trial
- PMID: 40526384
- PMCID: PMC12175031
- DOI: 10.1001/jamanetworkopen.2025.16318
Erenumab for Chronic Cluster Headache: A Randomized Clinical Trial
Abstract
Importance: Calcitonin gene-related peptide (CGRP) is involved in the pathophysiology of cluster headache (CH). Prophylactic pharmacologic treatment options for chronic CH (CCH) are limited. The potential effects of erenumab, a CGRP receptor antagonist monoclonal antibody, in treating CCH have not been assessed.
Objective: To evaluate the superiority of erenumab compared with placebo in the prophylaxis of CCH.
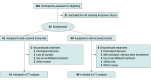
Design, setting, and participants: A 12-week, double-blind, placebo-controlled randomized clinical trial (CHERUB01) was conducted at 11 sites in Germany from December 2, 2021, to September 27, 2023. Participants (aged 18-65 years) had a diagnosis of CCH, had no previous sufficient response to standard CCH prophylactic medications approved in Germany, and had experienced at least 9 attacks during screening.
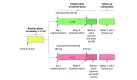
Intervention: Loading dose of erenumab (280 mg subcutaneously) or matching placebo in a 1:1 randomization, followed by another dose of erenumab (140 mg subcutaneously) or placebo 4 weeks later.
Main outcomes and measures: The primary end point was the reduction of mean weekly CH attacks from baseline over weeks 5 and 6. Key secondary end points were 50% responder rates and changes in Patient Global Impression of Improvement (PGI-I) scores. Safety and tolerability were also assessed. A bayesian analysis scheme was used for statistical analysis.
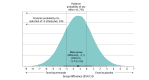
Results: This study randomized 81 participants (mean [SD] age, 48.9 [10.4] years; 60 men [74.1%]) with CCH (mean [SD], 21.5 [9.7] attacks per week) to erenumab (n = 41) or placebo (n = 40). Recruitment was stopped prematurely due to insufficient patient numbers meeting the inclusion criteria within the planned recruitment period. The primary end point was not met over weeks 5 and 6 of the double-blind phase because the mean (SD) reduction of weekly CH attacks was -7.3 (8.6) per week for erenumab and -5.9 (10.5) per week for placebo (group difference, -1.5 [95% credible interval [CrI], -5.7 to 2.8]). At weeks 5 and 6, the percentage of participants with a 50% or greater reduction in CH attacks was not significantly different between the erenumab (13 [31.7%]) and placebo (18 [45.0%]) groups (odds ratio, 0.5 [95% CrI, 0.2-1.5]). PGI-I scores were also not different between groups. More participants reported adverse events with erenumab than placebo (27 [65.9%] vs 17 [42.5%]), which were mostly of mild or moderate intensity.
Conclusions and relevance: In this clinical trial of patients with CCH, blockade of the CGRP receptor with erenumab was not successful in the prophylaxis of attacks. Future studies should revisit the role of CGRP in CCH.
Trial registration: ClinicalTrials.gov Identifier: NCT04970355; EudraCT Number: 2020-004399-16.
Conflict of interest statement
Figures
References
-
- Brandt RB, Wilbrink LA, de Coo IF, et al. ; ICON study group . A prospective open label 2-8 year extension of the randomised controlled ICON trial on the long-term efficacy and safety of occipital nerve stimulation in medically intractable chronic cluster headache. EBioMedicine. 2023;98:104895. doi: 10.1016/j.ebiom.2023.104895 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

