E3 ubiquitin ligase Listerin regulates macrophage cholesterol efflux and atherosclerosis by targeting ABCA1
- PMID: 40526435
- PMCID: PMC12352907
- DOI: 10.1172/JCI186509
E3 ubiquitin ligase Listerin regulates macrophage cholesterol efflux and atherosclerosis by targeting ABCA1
Abstract
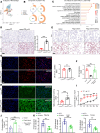
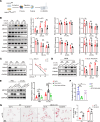
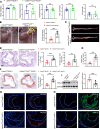
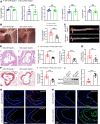
Atherosclerosis arises from disrupted cholesterol metabolism, notably impaired macrophage cholesterol efflux leading to foam cell formation. Through single-cell and bulk RNA-Seq, we identified Listerin E3 ubiquitin protein ligase 1 (Listerin) as a regulator of macrophage cholesterol metabolism. Listerin expression increased during atherosclerosis progression in humans and rodents. Its deficiency suppressed cholesterol efflux, promoted foam cell formation, and exacerbated plaque features (macrophage infiltration, lipid deposition, necrotic cores) in macrophage-specific KO mice. Conversely, Listerin overexpression attenuated these atherosclerotic manifestations. Mechanistically, Listerin stabilizes ABCA1, a key cholesterol efflux mediator, by catalyzing K63-linked polyubiquitination at residues K1884/K1957, countering ESCRT-mediated lysosomal degradation of ABCA1 induced by oxidized LDL (oxLDL). ABCA1 agonist erythrodiol restored cholesterol efflux in Listerin-deficient macrophages, while KO of ABCA1 abolished Listerin's effects in Tsuchiya human monocytic leukemia line (THP-1) cells. This study establishes Listerin as a protective factor in atherosclerosis via posttranslational stabilization of ABCA1, offering a potential therapeutic strategy targeting ABCA1 ubiquitination to enhance cholesterol efflux.
Keywords: Atherosclerosis; Cardiology; Metabolism; Ubiquitin-proteosome system.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

