CNS-targeted base editing of the major late-onset Tay-Sachs mutation alleviates disease in mice
- PMID: 40526437
- PMCID: PMC12352896
- DOI: 10.1172/JCI183434
CNS-targeted base editing of the major late-onset Tay-Sachs mutation alleviates disease in mice
Abstract
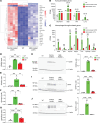
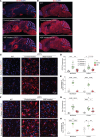
Late-onset Tay-Sachs (LOTS) disease is a lysosomal storage disorder most commonly caused by a point mutation (c.805G>A) in the HEXA gene encoding the α subunit of the lysosomal enzyme β-hexosaminidase A. LOTS manifests as a range of gradually worsening neurological symptoms beginning in young adulthood. Here, we explored the efficacy of an adenine base editor (ABE) programmed with an sgRNA to correct the HEXA c.805G>A mutation. Base editing in fibroblasts from a patient with LOTS successfully converted the pathogenic HEXA c.805A to G and partially restored β-hexosaminidase activity, with minimal genome-wide off-target editing. We generated a LOTS mouse model in which the mice exhibited decreased β-hexosaminidase activity, accumulation of GM2 ganglioside in the brain, progressive neurological manifestations, and reduced lifespan. Treatment of LOTS mice with the neurotropic virus AAV-PHP.eB carrying the ABE and an sgRNA targeting the LOTS point mutation partially corrected the c.805G>A mutation in the CNS, significantly increased brain β-hexosaminidase activity, and substantially reduced GM2 ganglioside accumulation in the brain. Moreover, the therapy delayed symptom onset and significantly extended median lifespan. These findings highlight the potential of base editing as an effective treatment for LOTS and its broader applicability to other lysosomal storage disorders.
Keywords: Genetic diseases; Genetics; Monogenic diseases; Neuroscience.
Conflict of interest statement
Figures





Similar articles
-
Generation of three human induced pluripotent stem cell (hiPSC) lines from patients with Late-Onset Tay-Sachs disease (HEXA-related adult-onset GM2-gangliosidosis).Stem Cell Res. 2025 Sep;87:103801. doi: 10.1016/j.scr.2025.103801. Epub 2025 Aug 9. Stem Cell Res. 2025. PMID: 40819571
-
Improvement of motor and behavioral activity in Sandhoff mice transplanted with human CD34+ cells transduced with a HexA/HexB expressing lentiviral vector.J Gene Med. 2020 Sep;22(9):e3205. doi: 10.1002/jgm.3205. Epub 2020 May 7. J Gene Med. 2020. PMID: 32335981 Free PMC article.
-
GM2 ganglioside accumulation causes neuroinflammation and behavioral alterations in a mouse model of early onset Tay-Sachs disease.J Neuroinflammation. 2020 Sep 20;17(1):277. doi: 10.1186/s12974-020-01947-6. J Neuroinflammation. 2020. PMID: 32951593 Free PMC article.
-
Advances in Diagnosis, Pathological Mechanisms, Clinical Impact, and Future Therapeutic Perspectives in Tay-Sachs Disease.Neurol Int. 2025 Jun 25;17(7):98. doi: 10.3390/neurolint17070098. Neurol Int. 2025. PMID: 40710901 Free PMC article. Review.
-
[Molecular pathogenesis and therapeutic approach of GM2 gangliosidosis].Yakugaku Zasshi. 2013;133(2):269-74. doi: 10.1248/yakushi.12-00199. Yakugaku Zasshi. 2013. PMID: 23370522 Review. Japanese.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

