Efficacy of NAMPT inhibition in T-cell acute lymphoblastic leukemia
- PMID: 40526635
- PMCID: PMC12173385
- DOI: 10.1371/journal.pone.0324443
Efficacy of NAMPT inhibition in T-cell acute lymphoblastic leukemia
Abstract
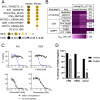
Novel agents targeting upregulated signaling pathways are needed to improve outcomes in T-cell acute lymphoblastic leukemia (T-ALL), since conventional cytotoxic chemotherapy regimens have reached the limits of tolerability. We identified upregulated, targetable signaling pathways common to both human T-ALL samples and a KrasLSL-G12D/+.Mb1Cre/+ murine model of T-ALL. We found the NAMPT inhibitor FK866 had the greatest cytotoxicity of a panel of small molecule inhibitors tested in human and mouse T-ALL cell lines, and in patient derived xenograft (PDX)-expanded T-ALL patient samples. We subsequently tested FK866 in vivo in PDX mouse models of T-ALL, and found that it significantly reduced the peripheral blood disease burden and prolonged the survival of leukemic mice (median survival of 60.5 vs 21 days, p = 0.0007). This screen for targetable pathways in T-ALL generated in vitro and in vivo preclinical data supporting NAMPT inhibition as a promising strategy for the treatment of T-ALL.
Copyright: © 2025 Vrana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

