Obstetric and newborn outcomes of mothers with and without HIV infection in Anaka general hospital in Northern Uganda
- PMID: 40526733
- PMCID: PMC12173179
- DOI: 10.1371/journal.pone.0326322
Obstetric and newborn outcomes of mothers with and without HIV infection in Anaka general hospital in Northern Uganda
Abstract
Background: Women of reproductive age constitute a significant proportion of the global HIV burden, with millions becoming pregnant while on lifelong antiretroviral therapy (ART). Although ART has dramatically improved maternal and child health outcomes, concerns persist regarding its safety in pregnancy. This study compares obstetric and neonatal outcomes between women with and without HIV infection at Anaka General Hospital, a rural hospital in northern Uganda.
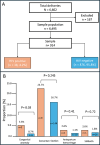
Methods: A hospital-based retrospective cross-sectional study at Anaka General Hospital in northern Uganda was conducted from July 2020 to June 2023. A total of 914 delivery records were included, sampled using systematic random sampling from the hospital maternity register. Data were extracted from maternity and neonatal records using a structured tool. Associations between HIV status and obstetric or neonatal outcomes were assessed using Chi-square or Fisher's exact tests for categorical variables and independent sample t-tests for continuous variables. Multivariable logistic regression was used to adjust for potential confounders and a p < 0.05 was considered statistically significant.
Results: Of the 914 participants included in the study 38 (4.2%) were HIV-positive. The odds of congenital anomalies were significantly higher among infants born to HIV-positive women compared with HIV-negative women (adjusted odds ratio = 9.76, 95% confidence interval: 1.72-55.48, p < 0.01). No significant differences were observed in maternal or neonatal outcomes between the two groups.
Conclusion: HIV infection was significantly associated with an increased risk of congenital anomalies, while other obstetric and neonatal outcomes were similar between HIV-positive and HIV-negative women. We recommend enhanced prenatal monitoring and early fetal screening among HIV-positive pregnant women on dolutegravir-based ART. Additionally, prospective studies are needed to better understand the contribution of dolutegravir and maternal factors to congenital anomalies in HIV-exposed pregnancies.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
References
-
- UNAIDS. UNAIDS Global AIDS Update 2022. 2022. https://www.unaids.org/sitess/default/files/media_asset/2022-global-aids...
-
- WHO. The use of antiretroviral drugs for treating and preventing HIV infection. 2016. [Cited 2024 July 13]. https://www.who.int/publications/i/item/9789241549684 - PubMed
-
- Smith CT, Megli C, Chappell CA. Infectious Diseases in Pregnancy. Obstetric Anesthesia Uncommon Disorders. 2023. p. 367.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical