Chemoselective Difluoromethylation of Nucleosides
- PMID: 40526795
- PMCID: PMC12210264
- DOI: 10.1021/acs.orglett.5c02204
Chemoselective Difluoromethylation of Nucleosides
Abstract
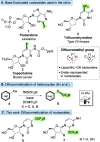
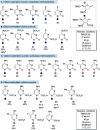
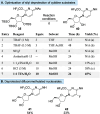
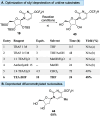
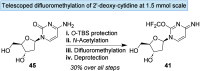
A profiling platform to define the chemoselectivity of the carbene-mediated difluoromethylation of nucleosides is described. First, the optimized reaction conditions for the difluoromethylation of silyl-protected nucleosides are established using TMS-CF2Br and KOAc to form the difluoromethyl carbene in situ. Second, the scope of these reaction conditions to difluoromethylate uridine- and cytidine-based 2'-deoxyribonucleoside and ribonucleoside analogues is established. When uridine analogues are substrates, O-difluoromethylation at the 4-position is observed, whereas O-difluoromethylation at the 2-position of cytidine analogues predominates. S-Difluoromethylation is preferable over O-difluoromethylation when thionucleosides are used. In all cases, no N-difluoromethylation is observed. Finally, silyl deprotection afforded difluoromethylated free nucleosides, thereby enabling the exploration of their utility for broader applications in medicinal chemistry and chemical biology.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

