Severe parvovirus B19 infection in patients with sickle cell disease hospitalized in intensive care units
- PMID: 40526829
- PMCID: PMC12657279
- DOI: 10.1182/bloodadvances.2025015947
Severe parvovirus B19 infection in patients with sickle cell disease hospitalized in intensive care units
Abstract
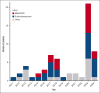
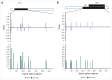
Parvovirus B19 infection can lead to severe complications in patients with chronic hemolysis. The aim of this study was to describe severe parvovirus B19 infections in adult patients with sickle cell disease (SCD). In this multicenter, retrospective, observational cohort study, adult patients with SCD admitted to intensive care units (ICUs) between 2011 and 2024 with acute parvovirus B19 infection were included. Unsupervised analysis was performed including clinical and biological characteristics to identify clusters of patients with different outcomes. Clinical phenotypes were defined based on patient clustering. Parvovirus B19 genomes from ICU (n = 15) and non-ICU control patients (n = 15) admitted to the hospital during the same period were sequenced and compared. Sixty-one patients (52% female; median age, 29 years [interquartile range, 24-38]) from 8 ICUs in France were included. Three clusters of patients were identified. From these clusters, 3 groups of patients with distinct clinical phenotype were identified: erythroblastopenia (n = 26), bone marrow necrosis (BMN) and fat cerebral embolism syndrome (CFE; n = 17), and other vaso-occlusive manifestations (n = 18). Length of stay in the ICU and hospital was longer in patients with BMN/CFE. There was no difference in parvovirus B19 genotype or NS1 or VP1/2 amino acid diversity between the groups. Similar results were observed between patients who were admitted to the ICU and those who were not. ICU patients with SCD and acute parvovirus B19 infection presented 3 clinical phenotypes associated with different initial severity and outcome but with similar parvovirus B19 clades and amino acid diversity.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.G. reports support from Pharma Dom for attending a meeting, outside the submitted work. S.F. has served as a speaker for GlaxoSmithKline, AstraZeneca, MSD, Pfizer, Cepheid, and Moderna. N.d.P. has served as an advisor or speaker for Moderna and AstraZeneca. The remaining authors declare no competing financial interests.
Figures



References
-
- Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350(6):586–597. - PubMed
-
- Anderson MJ, Higgins PG, Davis LR, et al. Experimental parvoviral infection in humans. J Infect Dis. 1985;152(2):257–265. - PubMed
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet Lond Engl. 2010;376(9757):2018–2031. - PubMed
-
- Serjeant GR, Serjeant BE, Thomas PW, Anderson MJ, Patou G, Pattison JR. Human parvovirus infection in homozygous sickle cell disease. Lancet Lond Engl. 1993;341(8855):1237–1240. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

