Real-world evidence of duvelisib and romidepsin in relapsed/refractory peripheral and cutaneous T-cell lymphomas
- PMID: 40526834
- PMCID: PMC12395046
- DOI: 10.1182/bloodadvances.2025016347
Real-world evidence of duvelisib and romidepsin in relapsed/refractory peripheral and cutaneous T-cell lymphomas
Abstract
Patients with relapsed or refractory (R/R) peripheral T-cell lymphomas (PTCL) require lineage-specific therapies to bridge to hematopoietic stem cell transplantation (HSCT). A previous phase 1/2 study of duvelisib/romidepsin (duv/romi) reported an overall response rate (ORR) of 58% and a complete response rate (CRR) of 42% with reduced grade 3 to 4 transaminitis (14%). We report real-world duv/romi outcomes in a multicenter, 38-patient R/R PTCL cohort. The median age at diagnosis was 62 years. Histological subtypes included nodal T follicular helper cell (nTFH; n = 17), PTCL-not otherwise specified (n = 14), cutaneous T-cell lymphoma (TCL; n = 3), extranodal natural killer/TCL (n = 1), ALK-negative anaplastic large cell lymphoma (n = 1), adult T-cell leukemia/lymphoma (n = 1), and hepatosplenic TCL (n = 1). The median previous therapy count was 1 (interquartile range [IQR], 1-2); 15 patients relapsed and 23 were refractory to prior treatment, including 8 prior HSCT (5 autologous, 3 allogeneic). After a median of 3 cycles (IQR, 2-4), ORR and CRR were 61% and 47%, respectively, with higher ORR (82% vs 43%) and CRR (71% vs 29%) in nTFH versus non-nTFH. The median progression-free survival and overall survival (HSCT-censored) were 11 and 16 months for nTFH, versus 3.3 and 8.3 months for non-nTFH. The median time to response was 1.9 months (IQR, 1.7-2.6), duration of response was 21 months, and time to next therapy was 17 months. After duv/romi, 11 patients bridged to allo-HSCT. Treatment was well tolerated; the most common grade 3 to 4 toxicities were lymphopenia (n = 15), neutropenia (n = 15), thrombocytopenia (n = 10), and transaminitis (n = 6), seldom leading to discontinuation (n = 4) or death (n = 1). These findings reinforce duv/romi's efficacy and bridging role to curative HSCT in high-risk R/R PTCL.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.N.S. reports research funding from Secura Bio and Daiichi Sankyo. E.J. reports research funding from Celgene, Merck, Pharmacyclics, and Hoffman-La Roche; honoraria from Merck, Daiichi Sankyo, Bristol Myers Squibb, and Bayer; and patents and royalties from UpToDate. S.J. reports consultancy for Mersana Therapeutics, Myeloid Therapeutics, SIRPant Immunotherapeutics, and Abcuro, Inc; research funding from SIRPant Immunotherapeutics, Abcuro, Inc, Daiichi Sankyo, and Acrotech Biopharma Inc; and membership on an entity’s board of directors or advisory committees for Mersana Therapeutics, Myeloid Therapeutics, SIRPant Immunotherapeutics, Abcuro, Inc, Secura Bio, Daiichi Sankyo, and CRISPR Therapeutics. The remaining authors declare no competing financial interests.
Figures

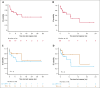
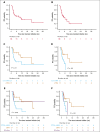
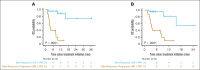
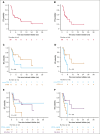
References
-
- Mehta-Shah N, Zinzani PL, Jacobsen ED, et al. Duvelisib in Patients with relapsed/refractory peripheral T-cell lymphoma: final results from the phase 2 PRIMO trial. Blood. 2024;144(suppl 1):3061.
-
- Horwitz SM, Moskowitz AJ, Mehta-Shah N, et al. The combination of duvelisib and romidepsin (DR) is highly active against relapsed/refractory peripheral T-cell lymphoma with low rates of transaminitis: final results. Hematol Oncol. 2021;39(S2)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

