Pralatrexate is effective in cytotoxic cutaneous T-cell lymphomas
- PMID: 40526835
- PMCID: PMC12345251
- DOI: 10.1182/bloodadvances.2025016680
Pralatrexate is effective in cytotoxic cutaneous T-cell lymphomas
Abstract
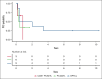
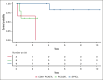
Cytotoxic cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of T-cell lymphomas with variable prognoses and no standard of care. We identified patients with primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma (CD8+ PCAETL), primary cutaneous γδ T-cell lymphoma (PCGDTL), and subcutaneous panniculitis-like T-cell lymphoma (SPTCL), who were treated with ≥1 dose of pralatrexate between 2015 and 2024 at the University of Washington/Fred Hutchinson Cancer Center. Eighteen patients met criteria, 3 with CD8+ PCAETL, 6 with PCGDTL, and 9 with SPTCL. The median number of prior systemic therapies was 1 (range, 0-4), and the median pralatrexate treatment duration was 14 (range, 8-43) weeks. The overall response rate was 100%, with 12 (67%) achieving complete response (CR). Median duration of progression-free survival and overall survival was 5.6 months and not reached, respectively. Among patients who achieved CR , the median response duration was 22 months. At a median follow-up of 45 months, 6 (33%) patients remain in sustained remission. This retrospective analysis is the first to evaluate pralatrexate's efficacy in this aggressive disease population, demonstrating its effectiveness and association with durable responses in cytotoxic CTCL.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
Subcutaneous Panniculitis-Like T-Cell Lymphoma With Increased γδ T Cells: A Potential Diagnostic Pitfall.Am J Dermatopathol. 2025 Jun 17;47(8):634-637. doi: 10.1097/DAD.0000000000002999. Am J Dermatopathol. 2025. PMID: 40532089
-
[Clinical Analysis of Primary Cutaneous CD8+ Aggressive Epidermotropic Cytotoxic T-Cell Lymphoma].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2025 Jun;33(3):777-783. doi: 10.19746/j.cnki.issn.1009-2137.2025.03.022. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2025. PMID: 40613169 Chinese.
-
Case series of long-term responders to pralatrexate in peripheral T-cell lymphoma.J Clin Exp Hematop. 2025;65(2):101-106. doi: 10.3960/jslrt.25013. J Clin Exp Hematop. 2025. PMID: 40582837 Free PMC article.
-
Chimeric antigen receptor (CAR) T-cell therapy for people with relapsed or refractory diffuse large B-cell lymphoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD013365. doi: 10.1002/14651858.CD013365.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515338 Free PMC article.
-
Evaluating mogamulizumab in the treatment of primary cutaneous T-cell lymphoma.Immunotherapy. 2025 Jun;17(8):551-559. doi: 10.1080/1750743X.2025.2520153. Epub 2025 Jun 24. Immunotherapy. 2025. PMID: 40552425 Review.
References
-
- Willemze R, Jansen PM, Cerroni L, et al. EORTC Cutaneous Lymphoma Group Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111(2):838–845. - PubMed
-
- Geller S, Myskowski PL, Pulitzer M, Horwitz SM, Moskowitz AJ. Cutaneous T-cell lymphoma (CTCL), rare subtypes: five case presentations and review of the literature. Chin Clin Oncol. 2019;8(1):5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous