IGSF9-targeted therapy inhibits the progression of acute myeloid leukemia
- PMID: 40526840
- PMCID: PMC12362688
- DOI: 10.1182/bloodadvances.2025016432
IGSF9-targeted therapy inhibits the progression of acute myeloid leukemia
Abstract
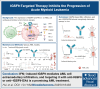
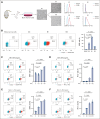
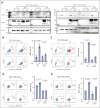
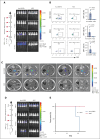
Previously, we reported that targeting immunoglobulin superfamily member 9 (IGSF9) could enhance antitumor T-cell activity and sensitivity to anti-PD-1 immunotherapy, although the detailed mechanism remains unclear. In this study, we find that, similar to the regulation of PD-L1 expression, interferon gamma (IFN-γ) also induces the expression of IGSF9 in acute myeloid leukemia (AML). The small interfering RNA specifically targeting JAK1 and a STAT1 inhibitor blocking IFN-γ signal pathway significantly inhibit the expression of IGSF9 and PD-L1. As a tumor-specific immune checkpoint molecule, IGSF9 plays a significant role in promoting tumor escape. The induction of both PD-L1 and IGSF9 by IFN-γ in the tumor microenvironment explains why IGSF9 is highly expressed in tumors and tumor-infiltrating immune cells. This induction also underpins the strong synergistic effects when combining anti-IGSF9 and anti-PD-1 therapies. Additionally, IGSF9 also mediates the extramedullary infiltration of AML cells, which can be inhibited by depletion of IGSF9 or anti-IGSF9. The binding epitopes of anti-IGSF9 are located within the immunoglobulin G2 and fibronectin type-III-2 domains of IGSF9. Based on these findings, we develop an antibody-drug conjugate (ADC) targeting IGSF9 (anti-IGSF9-linker-DXd). This ADC exhibits 99.7% purity, and primarily exists in monomeric form, demonstrating excellent homogeneity (drug-to-antibody ratio, 8-10) and specificity. Anti-IGSF9-linker-DXd effectively kills IGSF9-positive tumor cells and exhibits a potent bystander effect. In vivo, anti-IGSF9-linker-DXd almost completely eliminates early- and mid-stage tumors and significantly inhibits the progression of advanced tumors. In summary, our findings underscore the potential of IGSF9 as a novel therapeutic target for AML treatment, highlighting its role in disease progression and the efficacy of targeted therapies.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: Anti-IGSF9 and anti–IGSF9-linker-DXd have been patented (no. ZL202211000509.7 and 202311326074.X), which covers anti-IGSF9, anti–IGSF9-linker-DXd, and their application for treating tumors; and Z.L., Juan Zhang, Z.Z., S.J., and H.W. are listed as inventors. The remaining authors declare no competing financial interests.
Figures



Similar articles
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Byrd JC, Mrózek K, Dodge RK, et al. Cancer and Leukemia Group B (CALGB 8461) Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–4336. - PubMed
-
- Ganzel C, Manola J, Douer D, et al. Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: analysis of patients in ECOG-ACRIN Cancer Research Group Trials, 1980-2008 [published correction appears in J Clin Oncol. 2017;35(2):263] J Clin Oncol. 2016;34(29):3544–3553. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

