Minimum Data Set and Metadata for Active Vaccine Safety Surveillance: Systematic Review
- PMID: 40526902
- PMCID: PMC12187031
- DOI: 10.2196/63161
Minimum Data Set and Metadata for Active Vaccine Safety Surveillance: Systematic Review
Abstract
Background: Active vaccine safety surveillance (AVSS) stands as a top priority for the World Health Organization (WHO), serving as a critical indicator of the fourth maturity level for national regulatory agencies.
Objective: This review aims to define the minimal data scope for association studies in vaccine safety, providing a reference framework for implementing AVSS systems worldwide, especially in low- and middle-income countries.
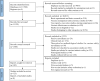
Methods: The study systematically searched PubMed, Embase, and Web of Science for cohort and case-control studies related to AVSS published between January 1, 2018, and September 7, 2022. Guided by the WHO and Council for International Organizations of Medical Sciences guidelines (CIOMS), we developed a 4D framework for Minimum Data Sets (MDSs), including "Vaccine," "Outcome," "Demographic Data," and "Covariate." Variables with a frequency of at least 5% were included in the MDS.
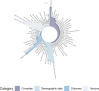
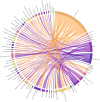
Results: Of the 123 included studies, 102 (82.9%) were cohort studies and 98 (79.7%) originated from high-income countries, covering populations across the entire life course. The MDS for COVID-19 vaccines identified 54 variables, while the MDS for maternal populations included 96 variables. WHO guidelines were found to align better with practical applications compared to CIOMS guidelines, though both require further optimization based on the MDS findings. However, metadata for these essential variables were inadequately described across the studies.
Conclusions: The proposed MDS provides clear guidance and concise requirements for AVSS data scope. Establishing a globally standardized MDS and comprehensive metadata based on these findings is essential to enhancing the global vaccine safety ecosystem.
Keywords: active surveillance; association; metadata; minimum data sets; vaccine safety.
© Mengdi Zhang, Junting Yang, Yan Li, Yuan Li, Tong Li, Ziqi Dong, Shuo Gong, Yahui Wu, Minrui Ren, Chunxiang Fan, Lina Zhang, Yi Wang, Yali Wang, Jingtian Ren, Feng Sun, Chuanyong Shen, Keli Li, Zhike Liu, Siyan Zhan. Originally published in JMIR Public Health and Surveillance (https://publichealth.jmir.org).
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

