Serotype 3 invasive pneumococcal disease in Tuscany across the eras of conjugate vaccines (2005-2024) and anthropic-driven respiratory virus fluctuations
- PMID: 40526905
- PMCID: PMC12184145
- DOI: 10.1080/21645515.2025.2510005
Serotype 3 invasive pneumococcal disease in Tuscany across the eras of conjugate vaccines (2005-2024) and anthropic-driven respiratory virus fluctuations
Abstract
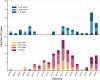
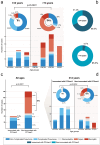
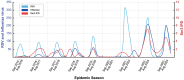
Serotype 3 Streptococcus pneumoniae (ser3) has emerged as a leading cause of invasive pneumococcal disease (IPD) despite targeted vaccination efforts. Preliminary evidence reported a differential impact of anti-ser3-pneumococcal-conjugate-vaccines (PCVser3) on different clinical presentations of ser3-IPD. Recently, a temporal association between respiratory syncytial virus (RSV) and IPD was observed in children, supporting a role of RSV in driving IPD dynamics. Ser3 IPD cases occurred in Tuscany from November 2005 to March 2024 were included. Comparisons of different clinical presentations (bacteremic pneumonias and osteomyelitis versus sepsis and meningitis) were made between younger and older patients and between vaccinated and unvaccinated ones. The temporal correlation between ser3 IPD, RSV and influenza was qualitatively described from 2015/2016 to 2023/2024. Among 160 ser3 IPD recorded cases, fully-immunized patients showed a significantly lower proportion of sepsis and meningitis compared to non-immunized patients (all ages: 7.5% versus 58.5%, p < .0001; aged 14-years and younger: 0% versus 31.3%, p = .0019). Ser3 IPD showed a strong temporal association with influenza virus outbreak but not with isolated RSV outbreak. Nearly two decades of molecular surveillance of ser3 in Tuscany suggest that the most severe IPD presentations, such as sepsis and meningitis, are significantly lower in individuals who received PCVser3 immunization. Ser3 IPD incidence is temporally associated with influenza outbreaks but not with RSV.
Keywords: Invasive pneumococcal disease; PCV; children; immunization; meningitis; pneumococcal conjugate vaccine; sepsis; serotype 3.
Conflict of interest statement
MG reports personal fees from Sanofi, Thermo Fisher Scientific.
Figures
References
-
- ’Fazio C, ’Camilli R, ’Giufrè M.. ISS-Sorveglianza Nazionale Delle Malattie Batteriche Invasive (MIB) 2020–2022.
-
- Andrejko KL, Gierke R, Rowlands JV, Rosen JB, Thomas A, Landis ZQ, Rosales M, Petit S, Schaffner W, Holtzman C, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease among children in the United States between 2010 and 2019: an indirect cohort study. Vaccine. 2024;42(16):3555–3563. doi: 10.1016/j.vaccine.2024.04.061. - DOI - PMC - PubMed
-
- Horácio AN, Silva-Costa C, Lopes JP, Ramirez M, Melo-Cristino J. Serotype 3 remains the leading cause of invasive pneumococcal disease in adults in Portugal (2012–2014) despite continued reductions in other 13-valent conjugate vaccine serotypes. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.01616. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
