Abatacept versus tocilizumab for the treatment of rheumatoid arthritis in TNF inhibitor inadequate responders: study protocol of the SUNSTAR randomised controlled open-label superiority trial
- PMID: 40527572
- PMCID: PMC12182147
- DOI: 10.1136/bmjopen-2024-098298
Abatacept versus tocilizumab for the treatment of rheumatoid arthritis in TNF inhibitor inadequate responders: study protocol of the SUNSTAR randomised controlled open-label superiority trial
Abstract
Introduction: Biological disease modifying antirheumatic drugs (bDMARDs) have a central role in the treatment of rheumatoid arthritis (RA). Tumour necrosis factor inhibitors (TNFis) are commonly used as first-line agents, while non-TNFis (tocilizumab, abatacept and rituximab) have shown to be non-inferior to TNFis in head-to-head trials. In case of TNFi inadequate response, using other mechanisms of action provides a better response than using an alternate TNFi. Which non-TNFi bDMARD administered subcutaneously to allow for ambulatory management to choose in case of first line TNFi inadequate response has not been tested in a randomised clinical trial, while observational data support a potential superiority of tocilizumab over abatacept.
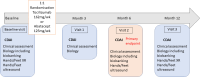
Methods and analysis: The SUNSTAR (SUbcutaNeouS Tocilizumab vs Abatacept in TNF Alpha inadequate responders for the treatment of Rheumatoid arthritis) study is a 52-week prospective, randomised, multicentre, open-label, superiority phase IV trial comparing subcutaneous tocilizumab with abatacept in a 1:1 ratio. Patients with active RA (Disease Activity Score-erythrocyte sedimentation rate >3.2 and Clinical Disease Activity Index (CDAI) >10) and with inadequate 3-month response to a first or second TNFi are included in 25 centres in France. The primary outcome is the CDAI improvement at week 24. Intention-to-treat analysis will be applied primarily. The secondary outcome is a composite outcome of the percentage of responders defined as a CDAI<10, no use of rescue treatment after week 12 and no experimental treatment change, at weeks 12, 24 and 52.
Ethics and dissemination: Ethics approval was obtained from the committee for the protection of persons (Comité de protection des personnes Sud Méditerranée I 17.00608.001744). The findings from this study, whether positive or negative, will be published in peer-reviewed journals and will be presented at national and international conferences. The results will inform future recommendations on the management of RA.
Trial registration number: NCT03227419 and EudraCT2017-000947-41.
Keywords: Clinical Trial; Rheumatology; THERAPEUTICS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical