Circulating histones as clinical biomarkers in critically ill conditions
- PMID: 40527588
- PMCID: PMC12643065
- DOI: 10.1002/1873-3468.70093
Circulating histones as clinical biomarkers in critically ill conditions
Abstract
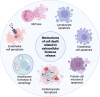
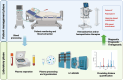
Extracellular histones, primarily nuclear proteins involved in chromatin organization, have emerged as key mediators in pathological processes in critically ill patients. When released into circulation due to cell death mechanisms such as NETosis, histones act as damage-associated molecular patterns (DAMPs), contributing to excessive inflammation, endothelial dysfunction, immune response dysregulation, coagulation activation, cell death, and multi-organ damage. Increasing evidence supports their role in the pathophysiology of sepsis, acute lung injury, cardiac injury, pancreatitis, and other life-threatening conditions. Given their strong association with disease severity and prognosis, circulating histones have gained attention as potential clinical biomarkers for early diagnosis, prognosis, and therapeutic monitoring in critically ill patients. This review discusses the biological roles of extracellular histones, their potential as biomarkers, different approaches to measure them, and emerging therapeutic strategies aimed at neutralizing or removing circulating histones to improve patient outcomes in severe medical conditions. Impact statement This review highlights extracellular histones as key mediators and biomarkers in sepsis, proposing their use in diagnosis, prognosis, and treatment monitoring. Integrating quantitative proteomics for the detection of circulating histones may enhance patient stratification and guide therapeutic strategies, advancing personalized medicine in critical care.
Keywords: COVID‐19; acute kidney injury; cardiac injury; critically‐ill patient; extracellular histones; lung injury; mass spectrometry; pancreatitis; renal replacement therapy; sepsis; septic shock.
© 2025 The Author(s). FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
JLG‐G is co‐founder and owns shares of EpiDisease S.L., a Spin‐Off of the Consortium Center for Biomedical Network Research of the ISCIII. EpiDisease S.L. has licensed the patent titled “Mass spectrometry‐based methods for the detection of circulating histones H3 and H2B in plasma from sepsis or septic shock (ss) patients” with reference number EP3535587B1, and extended to USA, China, Japan, and Hong Kong. The remaining authors declare no conflict of interest related to this work.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

