Quantitative Analysis With Time-Dependent Diffusion MRI for Assessing WHO/ISUP Tumor Grade in Clear Cell Renal Cell Carcinoma
- PMID: 40527738
- PMCID: PMC12235544
- DOI: 10.3348/kjr.2024.1202
Quantitative Analysis With Time-Dependent Diffusion MRI for Assessing WHO/ISUP Tumor Grade in Clear Cell Renal Cell Carcinoma
Abstract
Objective: To investigate the feasibility of time-dependent diffusion-weighted imaging (td-dMRI) in assessing the pathological World Health Organization/International Society of Urological Pathology (WHO/ISUP) grade of clear cell renal cell carcinoma (ccRCC).
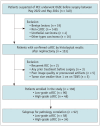
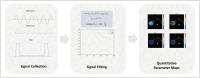
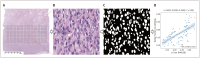
Materials and methods: A total of 138 patients (median age, 58 years [interquartile range, 51-64 years]; 89 males) with surgically confirmed ccRCC, comprising 48 high-grade (WHO/ISUP grade III/IV) and 90 low-grade (WHO/ISUP grade I/II) tumors, were included in the study among patients who underwent preoperative td-dMRI for suspected RCC between May 2022 and May 2024. The td-dMRI microstructural parameters, including cell diameter (d), intracellular volume fraction (fin), cellularity, and extracellular diffusivities (Dex), were quantified using a two-compartment model. The solid tumor area was manually annotated to extract the mean values from each parameter map. We analyzed the differences in td-dMRI parameters between the high- and low-grade tumors and evaluated the ability of these parameters to distinguish between the two tumor groups. High-definition hematoxylin-and-eosin-stained slides were obtained from 92 patients. We assessed the correlation between td-dMRI parameters and pathologic nuclear fraction, which was quantified using an automated nucleus segmentation model (Hover-Net).
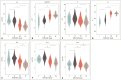
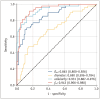
Results: Compared to high-grade tumors, low-grade tumors exhibited lower cellularity and fin and higher diameter and Dex. For differentiation between low- and high-grade ccRCC, the fin exhibited the highest diagnostic performance (areas under the receiver operating characteristic curve [AUC] = 0.943; 95% confidence interval, 0.906-0.980), followed by cellularity (AUC = 0.931; 0.887-0.976), Dex (AUC = 0.863; 0.800-0.926), and diameter (AUC = 0.690; 0.596-0.784). The nuclei on pathology slides were automatically segmented, and the nuclear fraction exhibited a moderate correlation with fin (r = 0.65, P < 0.001).
Conclusion: td-dMRI parameters show potential for assessing pathological WHO/ISUP grades and may serve as promising noninvasive biomarkers for characterizing RCC.
Keywords: Diffusion weighted image; Renal cell carcinoma; Time-dependent diffusion; WHO/ISUP grade.
Copyright © 2025 The Korean Society of Radiology.
Conflict of interest statement
The authors of this manuscript declare relationships with the following company: Philips Healthcare. One of our authors (Xiaoyong Zhang) is an employee of Philips Healthcare. This author only provided technical support and did not have control over the data at any point during the study.
Figures

References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. - PubMed
-
- Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82:529–542. - PubMed
-
- Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Ljungberg B, et al. The 2022 updated European Association of Urology guidelines on the use of adjuvant immune checkpoint inhibitor therapy for renal cell carcinoma. Eur Urol. 2023;83:10–14. - PubMed
-
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

