Single-Cell Analysis Reveals Fibroblast-Derived Migrasomes as CXCL12 Carriers Promoting Skin Wound Repair
- PMID: 40527746
- PMCID: PMC12173532
- DOI: 10.1002/jev2.70112
Single-Cell Analysis Reveals Fibroblast-Derived Migrasomes as CXCL12 Carriers Promoting Skin Wound Repair
Abstract
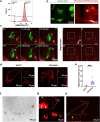
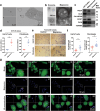
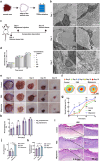
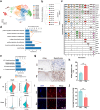
Migrasomes are newly discovered organelles with demonstrated functions in organ morphogenesis and angiogenesis. However, the effect of migrasomes in tissue repair remains unreported. Our super-resolution confocal microscopy and focused ion beam scanning electron microscopy results confirmed that migrasomes were directly connected with retraction fibres and could release their contents into the surroundings in human and rat skins and oral mucosae. Multiplex immunofluorescence staining results revealed that these retraction fibres and migrasomes originated from fibroblasts. Live-cell imaging demonstrated that human oral mucosal fibroblast-derived migrasomes could be taken up by both fibroblasts and HaCaT cells. In addition, the injection of purified fibroblast-derived migrasomes into the edges of rat skin wounds significantly accelerated wound healing. Single-cell sequencing results suggested that the clusters of keratinocytes, fibroblasts, and endothelial cells play key roles in the wound-healing process. Moreover, the expression of Vegfa, Il-6, and Col1a1 in the fibroblast subcluster was significantly upregulated. Furthermore, these purified migrasomes increased the protein levels of VEGFA, IL-6, and COL1A1 in cultured fibroblasts in vitro. Mechanistically, migrasomes may facilitate wound healing by delivering CXCL12. Thus, our research revealed that fibroblast-derived migrasomes are potential therapeutic vesicles for skin wound-healing repair.
Keywords: extracellular vesicles; migrasome; single‐cell RNA sequence; skin wound healing.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Young fibroblast-derived migrasomes alleviate keratinocyte senescence and enhance wound healing in aged skin.J Nanobiotechnology. 2025 Mar 11;23(1):200. doi: 10.1186/s12951-025-03293-2. J Nanobiotechnology. 2025. PMID: 40069826 Free PMC article.
-
Human-induced pluripotent stem cell-derived exosomes promote skin wound healing through activating FGF2-mediated p38 pathway.Mol Cell Biochem. 2025 Jul;480(7):4227-4242. doi: 10.1007/s11010-025-05244-9. Epub 2025 Mar 11. Mol Cell Biochem. 2025. PMID: 40064791
-
SPRR1B+ keratinocytes prime oral mucosa for rapid wound healing via STAT3 activation.Commun Biol. 2024 Sep 16;7(1):1155. doi: 10.1038/s42003-024-06864-5. Commun Biol. 2024. PMID: 39300285 Free PMC article.
-
The biogenesis and biological roles of migrasomes in human diseases.Cell Death Discov. 2025 Jul 1;11(1):296. doi: 10.1038/s41420-025-02569-8. Cell Death Discov. 2025. PMID: 40595452 Free PMC article. Review.
-
Migrasomes: a promising extracellular vesicle-like novel organelle for bone regeneration.Regen Med. 2025 Jul;20(7):305-316. doi: 10.1080/17460751.2025.2546213. Epub 2025 Aug 12. Regen Med. 2025. PMID: 40792636 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

