PubMed knowledge graph 2.0: Connecting papers, patents, and clinical trials in biomedical science
- PMID: 40527887
- PMCID: PMC12174349
- DOI: 10.1038/s41597-025-05343-8
PubMed knowledge graph 2.0: Connecting papers, patents, and clinical trials in biomedical science
Abstract
Papers, patents, and clinical trials are essential scientific resources in biomedicine, crucial for knowledge sharing and dissemination. However, these documents are often stored in disparate databases with varying management standards and data formats, making it challenging to form systematic and fine-grained connections among them. To address this issue, we construct PKG 2.0, a comprehensive knowledge graph dataset encompassing over 36 million papers, 1.3 million patents, and 0.48 million clinical trials in the biomedical field. PKG 2.0 integrates these dispersed resources through 482 million biomedical entity linkages, 19 million citation linkages, and 7 million project linkages. The construction of PKG 2.0 wove together fine-grained biomedical entity extraction, high-performance author name disambiguation, multi-source citation integration, and high-quality project data from the NIH Exporter. Data validation demonstrates that PKG 2.0 excels in key tasks such as author disambiguation and biomedical entity recognition. This dataset provides valuable resources for biomedical researchers, bibliometric scholars, and those engaged in literature mining.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


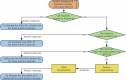
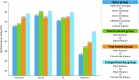


Similar articles
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article.
-
Manipulative therapies for infantile colic.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD004796. doi: 10.1002/14651858.CD004796.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235617 Free PMC article.
-
Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis.Cochrane Database Syst Rev. 2016 Oct 4;10(10):CD011812. doi: 10.1002/14651858.CD011812.pub2. Cochrane Database Syst Rev. 2016. PMID: 27699783 Free PMC article.
References
-
- Van Noorden, R., Maher, B. & Nuzzo, R. The top 100 papers. Nature News514(7524), 550 (2014). - PubMed
-
- Rowland, F. The peer‐review process. Learned publishing15(4), 247–258 (2002).
-
- Shatz, D. Peer review: A critical inquiry. Rowman & Littlefield. (2004).
-
- Reinhart, M. & Schendzielorz, C. Peer-review procedures as practice, decision, and governance—the road to theories of peer review. Science and Public Policy51(3), 543–552 (2024).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

