Efficacy and Safety of Allisartan Isoproxil/Amlodipine in Patients with Essential Hypertension: A Phase III, Multicenter, Double-Blind, Parallel-Group, Randomised study
- PMID: 40527906
- PMCID: PMC12343297
- DOI: 10.1038/s41371-025-01035-3
Efficacy and Safety of Allisartan Isoproxil/Amlodipine in Patients with Essential Hypertension: A Phase III, Multicenter, Double-Blind, Parallel-Group, Randomised study
Abstract
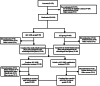
This multicenter, double-blind, parallel-group, randomised controlled phase III study evaluated the efficacy and safety of the Allisartan Isoproxil 240 mg /Amlodipine 5 mg (ALI/AML) combination compared with ALI 240 mg monotherapy in patients with mild-to-moderate essential hypertension. Patients aged 18 to 70 years with mean sitting systolic blood pressure (msSBP) between 140 and <180 mmHg and mean sitting diastolic blood pressure (msDBP) between 90 and <110 mmHg were randomised 1:1 to receive ALI/AML or ALI once-daily for 12 weeks after a 4-week treatment with ALI, followed by an open-label extension period with ALI/AML up to week 52. A total of 199 patients were randomised (ALI/AML: n = 99, ALI: n = 100) with 169 completing the study. Baseline characteristics were comparable between groups. After 12 weeks of randomisation, the reduction in msSBP (primary endpoint) was significantly greater in the ALI/AML group vs the ALI group (-18.3 vs. -9.3 mmHg, p < 0.001). Reductions in msDBP (-6.0 vs. -1.9 mmHg, p < 0.001) and 24-hour mean ambulatory systolic/diastolic blood pressure (-19.9/-10.1 vs. -6.9/-4.2 mmHg) were more pronounced in the ALI/AML group. Additionally, a greater proportion of patients achieved the BP response and target office BP in the ALI/AML group compared to the ALI group (53.6% vs. 25.5%, p < 0.001; 40.2% vs. 20.4%, p=0.0026). AML/ALI combination was generally safe and well tolerated, with sustained efficacy up to 52 weeks. The study concluded that ALI/AML offers a convenient, single-pill option for effective BP reduction in hypertensive patients.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Xiaojuan Lian, Yuanyuan Chen, Yan Chen, Hao Tang, Zichen Liu, and Peng Mi are employees of Shenzhen Salubris Pharmaceuticals Co., LTD. Other authors declare no conflict of interest. Ethics Approval and Consent to Participate: This study was approved by the Ethics Committee of the Beijing Chaoyang Hospital affiliated with the Capital Medical University(No. 2020-Medicine-89-3); prior to study enrollment and any of the study procedures, written informed consent was obtained from all subjects. Consent for Publication: All authors critically reviewed and approved the publication of the final paper.
Figures





Similar articles
-
Efficacy and Safety of Allisartan Isoproxil/Amlodipine in Patients With Essential Hypertension Uncontrolled by Amlodipine: A Phase III, Multicenter, Double-Blind, Parallel-Group, Randomized Controlled Trial.J Clin Hypertens (Greenwich). 2025 Jan;27(1):e14955. doi: 10.1111/jch.14955. J Clin Hypertens (Greenwich). 2025. PMID: 39821945 Free PMC article. Clinical Trial.
-
Efficacy and safety of a single-pill versus free combination of perindopril/indapamide/amlodipine: a multicenter, randomized, double-blind study in Chinese patients with hypertension.J Hypertens. 2024 Aug 1;42(8):1373-1381. doi: 10.1097/HJH.0000000000003741. Epub 2024 Apr 24. J Hypertens. 2024. PMID: 38660708 Free PMC article. Clinical Trial.
-
Efficacy and Tolerability of Combination Therapy Versus Monotherapy with Candesartan and/or Amlodipine for Dose Finding in Essential Hypertension: A Phase II Multicenter, Randomized, Double-blind Clinical Trial.Clin Ther. 2017 Aug;39(8):1628-1638. doi: 10.1016/j.clinthera.2017.06.014. Epub 2017 Jul 19. Clin Ther. 2017. PMID: 28734660 Clinical Trial.
-
Pharmacotherapy for hyperuricemia in hypertensive patients.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD008652. doi: 10.1002/14651858.CD008652.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Apr 13;4:CD008652. doi: 10.1002/14651858.CD008652.pub3. PMID: 23440832 Updated.
-
Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension.Cochrane Database Syst Rev. 2014 May 29;2014(5):CD003824. doi: 10.1002/14651858.CD003824.pub2. Cochrane Database Syst Rev. 2014. PMID: 24869750 Free PMC article.
References
-
- Hypertension Branch of Chinese Geriatrics Society, Beijing Hypertension Association, National Clinical Research Center of the Geriatric Diseases, Hua Q, Fan L, Wang ZW, Li J. 2023 Guideline for the management of hypertension in the elderly population in China. J Geriatr Cardiol. 2024;21:589–630. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

