Impact of stereochemical replacement on activity and selectivity of membrane-active antibacterial and antifungal cyclic peptides
- PMID: 40527928
- PMCID: PMC12174330
- DOI: 10.1038/s44259-025-00121-3
Impact of stereochemical replacement on activity and selectivity of membrane-active antibacterial and antifungal cyclic peptides
Abstract
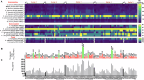
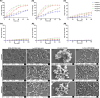
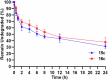
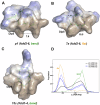
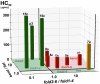
Herein, we report a library of 7-mer macrocyclic peptides designed by systematically replacing one, multiple, or all L-amino acids with their D-isomers in our previously identified hit compounds. Lead peptides, 15c and 16c, showed broad-spectrum activity against bacteria (Gram-positive minimum inhibitory activity (MIC 1.5-6.2 µg/mL and Gram-negative MIC 6.2-25 µg/mL) and fungi (MIC = 3.1-25 µg/mL). Additionally, peptides 15c and 16c showed rapid kill kinetics and biofilm degradation potential against both bacteria and fungi, while resistance development was not observed. The antimicrobial effect of these macrocyclic peptides was attributed to their membranolytic action, which was confirmed by calcein dye leakage assay and scanning electron microscopy analysis. Both peptides, 15c (HC50 = 335 µg/mL) and 16c (HC50 = 310 µg/mL), exhibited significantly lower hemolytic activity compared to their parent peptide p3 (HC50 = 230 µg/mL). At 100 µg/mL, both peptides showed >90% cell viability after 24 h incubation across four normal mammalian cell lines. Both peptides showed plasma stability (t1/2 ≥ 6 h), further supporting their therapeutic potential. Finally, the molecular mechanisms determining the pharmacological properties of a number of typical representatives of each series of synthesized peptides were investigated by NMR spectroscopy and computer simulations. The study revealed specific combinations of structural, dynamic, and hydrophobic parameters of these amphiphilic peptides that allow a reasonable prediction of their hemolytic activity. This Structure-Activity Relationship provides a basis for the rational design of peptides or peptidomimetics with predefined pharmacological profiles.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Roberts, R. R. et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin. Infect. Dis.49, 1175–1184 (2009). - PubMed
-
- Hancock, R. & Patrzykat, A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Targets Infect. Disord.2, 79–83 (2002). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

