RAMP1-dependent hormonal regulation of CGRP and its receptor in the trigeminal ganglion
- PMID: 40528180
- PMCID: PMC12172354
- DOI: 10.1186/s10194-025-02071-7
RAMP1-dependent hormonal regulation of CGRP and its receptor in the trigeminal ganglion
Abstract
Objectives: Menstrual migraine (MM) is a debilitating neurological disorder triggered by fluctuations in ovarian hormones, particularly estrogen. While the calcitonin gene-related peptide (CGRP) system is central to migraine pathophysiology, the molecular mechanisms linking hormonal changes to CGRP signaling remain unclear. This study investigates how sex hormones regulate CGRP-related gene expression in the trigeminal ganglion (TG), with a focus on the receptor activity-modifying protein 1 (RAMP1), using both wild-type (WT) and Ramp1 knockout (KO) mice.
Methods: We analyzed gene expression in the TG across the estrous cycle and following systemic estrogen or progesterone administration. Expression of Ramp1, Calca (encoding CGRPα), Calcrl (encoding CRLR), estrogen receptors, and related genes was assessed by RT-qPCR. WT and Ramp1 KO mice of both sexes were used to explore hormone-dependent gene regulation and RAMP1’s functional role.
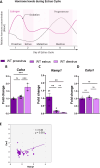
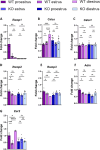
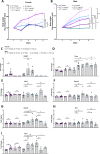
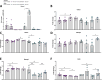
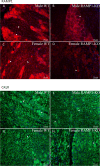
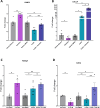
Results: Ramp1 expression varied across the estrous cycle, peaking in proestrus and declining in diestrus, inversely correlated with Calca. Calcrl levels remained unchanged. Ramp1 expression correlated significantly with Esr2 (encoding ERβ), suggesting estrogen receptor–mediated regulation. Estrogen treatment upregulated Ramp1 in both sexes; Calca was downregulated in females but upregulated in males. Progesterone had more modest effects, primarily altering Ramp3 expression. In Ramp1 KO mice, the cyclical variation of Calca, Ramp2, and Ramp3 seen in WT mice was absent, and basal Calca expression was elevated in males, indicating that RAMP1 is essential for hormonal regulation of the CGRP system. Additionally, our findings support a role for estrogen-driven epigenetic mechanisms, such as DNA methylation, in the long-term regulation of Ramp1.
Conclusion: This study highlights RAMP1 as a key mediator linking hormonal fluctuations to CGRP signaling in the TG. Hormone-dependent gene expression changes were sex-specific and disrupted in Ramp1 KO mice, supporting its role in migraine susceptibility. These findings provide mechanistic insight into hormonal migraine and suggest that both acute hormone signaling and long-term epigenetic regulation shape individual sensitivity to CGRP-based therapies.
Supplementary Information: The online version contains supplementary material available at 10.1186/s10194-025-02071-7.
Keywords: Calca; Migraine; Ramp1; Sex hormones; Trigeminal ganglion.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal part of the study followed the guidelines of the European Communities Council (86/609/ECC) and was approved by the Danish Animal inspectorate, license number 2023-25-0201-01469 and 2024-15-00202-00213. All animal experiments were therefore performed in accordance with the European Community Council Directive on ‘The Protection of Animals Used for Scientific Purposes’ (2010/63/EU). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Ex vivo stimulation of the trigeminal nucleus caudalis induces peripheral CGRP release in the trigeminal ganglion and reveals a distinct dopamine-endocannabinoid mechanism relevant to migraine.J Headache Pain. 2025 Jun 16;26(1):141. doi: 10.1186/s10194-025-02072-6. J Headache Pain. 2025. PMID: 40524163 Free PMC article.
-
Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion.Neuroscience. 2010 Aug 25;169(2):683-96. doi: 10.1016/j.neuroscience.2010.05.016. Epub 2010 May 22. Neuroscience. 2010. PMID: 20472035
-
Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine.Headache. 2011 May;51(5):674-92. doi: 10.1111/j.1526-4610.2011.01882.x. Headache. 2011. PMID: 21521205 Free PMC article.
-
CGRP receptor antagonism and migraine therapy.Curr Protein Pept Sci. 2013 Aug;14(5):386-92. doi: 10.2174/13892037113149990055. Curr Protein Pept Sci. 2013. PMID: 23745702 Review.
-
The Trigeminovascular Pathway: Role of CGRP and CGRP Receptors in Migraine.Headache. 2017 May;57 Suppl 2:47-55. doi: 10.1111/head.13081. Headache. 2017. PMID: 28485848 Review.
References
-
- Vetvik KG, MacGregor EA (2017) Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 16:76–87. 10.1016/s1474-4422(16)30293-9 - PubMed
-
- MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Incidence of migraine relative to menstrual cycle phases of rising and falling Estrogen. Neurology 67:2154–2158. 10.1212/01.wnl.0000233888.18228.19 - PubMed
-
- Lipton RB et al (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349. 10.1212/01.wnl.0000252808.97649.21 - PubMed
-
- Krause DN, Warfvinge K, Haanes KA, Edvinsson L (2021) Hormonal influences in migraine - interactions of oestrogen, Oxytocin and CGRP. Nat Rev Neurol 17:621–633. 10.1038/s41582-021-00544-2 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

