The pasteurized Weissella cibaria alleviates sepsis-induced acute lung injury by modulation of intestinal mucus barrier and gut microbiota
- PMID: 40528192
- PMCID: PMC12172371
- DOI: 10.1186/s12967-025-06674-1
The pasteurized Weissella cibaria alleviates sepsis-induced acute lung injury by modulation of intestinal mucus barrier and gut microbiota
Abstract
Background: Dysbiosis of intestinal microecology caused by sepsis plays a crucial role in the onset and progression of sepsis-induced acute lung injury (SALI). As a postbiotic type, inactivated probiotic bacteria can regulate the gut microbiome. Pasteurized bacteria are considered safer than live bacteria in immune dysregulation disorders. Weissella cibaria (W. cibaria) is considered a candidate probiotic with certain beneficial functions. However, whether inactivated W. cibaria can alleviate SALI and the underlying mechanisms remain unclear. This study aimed to investigate whether inactivated W. cibaria can regulate intestinal mucosal barrier function and gut microbiota, thereby improving SALI.
Methods: Following gavage of pasteurized W. cibaria in septic mice, lung tissue damage and inflammation levels were assessed. Circulating LPS levels and inflammatory cytokine concentrations in the blood were measured. Additionally, colonic tissue inflammation, intestinal mucosal barrier integrity, and alterations in the gut microbiota were evaluated.
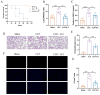
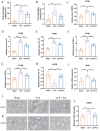
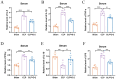
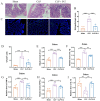
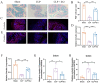
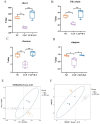
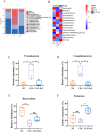
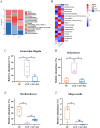
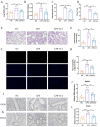
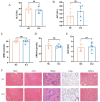
Result: Pasteurized W. cibaria increases survival rates in SALI mice and improves pathological damage and cell apoptosis in lung tissue. Pasteurized W. cibaria also reduces the lung inflammatory response in septic mice by lowering pro-inflammatory cytokine levels and increasing anti-inflammatory cytokine levels. Pasteurized W. cibaria appears to exert its effects by improving the intestinal mucosal barrier and reversing gut microbiota dysbiosis caused by sepsis. Specifically, pasteurized W. cibaria alleviates intestinal barrier damage and inflammation in SALI mice, enhancing the integrity of the intestinal mucosal barrier. Additionally, pasteurized W. cibaria increases the abundance of anti-inflammatory bacteria such as Muribaculaceae. Pasteurized W. cibaria also decreases the levels of LPS-producing bacteria, including Escherichia-Shigella and Helicobacter, leading to significant attenuation in metabolic endotoxemia, which in turn alleviates excessive lung inflammation in septic mice.
Conclusions: Pasteurized W. cibaria has the potential to act as a postbiotic agent, improving sepsis-induced gut microbiota dysbiosis and acute lung injury, and providing a novel strategy for treating SALI.
Keywords: Weissella cibaria; Gut microbiota; Intestinal mucosal barrier; Postbiotic; Sepsis-induced acute lung injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Institutional Animal Care and Use Committee of Zhengzhou University (approval number 2022-KY-1095-003). Consent for publication: All participants provided written informed consent to participate in the studies and for publication of the findings of the study. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Weissella cibaria suppresses colitis-associated colorectal cancer by modulating the gut microbiota-bile acid-FXR axis.mSystems. 2025 Jul 22;10(7):e0028825. doi: 10.1128/msystems.00288-25. Epub 2025 Jul 3. mSystems. 2025. PMID: 40607831 Free PMC article.
-
Gut microbiota-derived Proline-Leucine dipeptide aggravated sepsis-induced acute lung injury via activating Nod2/NF-κB signaling pathway.Mol Immunol. 2025 Aug;184:199-212. doi: 10.1016/j.molimm.2025.05.024. Epub 2025 Jul 4. Mol Immunol. 2025. PMID: 40614662
-
Osteopontin protects from ovalbumin-induced asthma by preserving the microbiome and the intestinal barrier function.mSystems. 2025 Jun 17;10(6):e0038925. doi: 10.1128/msystems.00389-25. Epub 2025 May 22. mSystems. 2025. PMID: 40401951 Free PMC article.
-
Radiation-induced injury and the gut microbiota: insights from a microbial perspective.Therap Adv Gastroenterol. 2025 Jun 16;18:17562848251347347. doi: 10.1177/17562848251347347. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40535532 Free PMC article. Review.
-
Microbiota-dependent modulation of intestinal anti-inflammatory CD4+ T cell responses.Semin Immunopathol. 2025 Apr 1;47(1):23. doi: 10.1007/s00281-025-01049-6. Semin Immunopathol. 2025. PMID: 40167791 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

