Interleukin-1β mediates a tumor-supporting environment prompted by IGF1 in triple-negative breast cancer (TNBC)
- PMID: 40528195
- PMCID: PMC12172323
- DOI: 10.1186/s12967-025-06730-w
Interleukin-1β mediates a tumor-supporting environment prompted by IGF1 in triple-negative breast cancer (TNBC)
Abstract
Background: The intricate mechanisms that associate obesity with triple-negative breast cancer (TNBC) remain to be disclosed. Considering that obesity is linked with an increased bioavailability of the insulin-like growth factor 1 (IGF1), we evaluated whether IGF1 triggers aggressive features in TNBC cells and the molecular paths involved.
Methods: Gene expression and Chromatin Immunoprecipitation experiments, ELISA, immunoblotting, immunoprecipitation and immunofluorescence assays, combined with two-dimensional and three-dimensional in vitro model-based studies, were used to investigate the molecular mechanisms through which IGF1 may promote proliferative and motile responses in TNBC cells and reprogram normal fibroblasts into cancer-associated fibroblasts (CAFs)-like cells. The translational relevance of the results obtained was supported by bioinformatics analyses leveraging data from extensive TNBC patient databases.
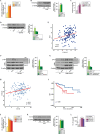
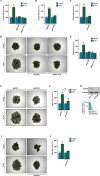
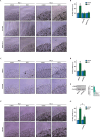
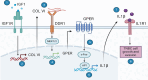
Results: We found that the cytokine interleukin-1β (IL-1β) mediates certain IGF1 actions within the tumor microenvironment, hence facilitating the TNBC landscape. Mechanistically, we assessed that the IGF1/IGF1 receptor (IGFIR) axis induces the collagen VI-dependent activation of discoidin domain receptor 1 (DDR1) and the subsequent increase of the G protein estrogen receptor (GPER), toward IL-1β regulation and secretion. Consequently, IL-1β promoted both the autocrine stimulation of TNBC cells and the differentiation of normal fibroblasts into cancer-associated fibroblasts (CAFs)-like cells, which in turn achieved a proliferative profile and enhanced the motility of TNBC cells.
Conclusions: IL-1β may be considered as a therapeutic target in more comprehensive approaches in obese TNBC patients exhibiting high IGF-1 bioavailability.
Keywords: Cancer associated fibroblasts (CAFs); Discoidin domain receptor 1 (DDR1); G protein estrogen receptor (GPER); Insulin-like growth factor 1 (IGF1); Interleukin 1β (IL1β); Triple-negative breast cancer (TNBC).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

