Impact of environmental microplastic exposure on HepG2 cells: unraveling proliferation, mitochondrial dynamics and autophagy activation
- PMID: 40528208
- PMCID: PMC12172363
- DOI: 10.1186/s12989-025-00632-x
Impact of environmental microplastic exposure on HepG2 cells: unraveling proliferation, mitochondrial dynamics and autophagy activation
Abstract
The rise of microplastic (MPs) pollution presents a pressing environmental issue, raising concerns about its potential health impacts on human populations. Given the critical role of the liver in detoxification and metabolism, understanding the effects of MPs on the human hepatoma cell line HepG2 cells is essential for comprehensively assessing the dangers associated with MPs pollution to human health. Until now, the assessment of the harmful impact of polyethylene (PE) and polyethylene terephthalate (PET) on HepG2 has been incomplete and lacks certain essential data points. In this particular setting, we examined parameters such as cell viability, oxidative stress, mtDNA integrity, mitochondrial membrane potential, and autophagy in HepG2 cells exposed for 72 h to PET and PE at a concentration of 10 µg/mL. Our data revealed that exposure of HepG2 to MPs causes an increase in cell viability accompanied by a heightened ROS and altered mitochondrial function, as revealed by decreased mtDNA integrity and membrane potential. In addition, results demonstrated that exposure to PET and PE activated autophagic events, as suggested by the increased levels of the specific markers LC3 and p62. This last point was further confirmed using bafilomycin, a specific blocker that hinders the merging of autophagosomes and lysosomes, thereby blocking autophagic degradation processes. Given the increasing evidence of food chain MPs contamination and its possible harmful effects, our data should be carefully considered.
Keywords: Bafilomycin A1; Hepatocellular carcinoma cell line; Macroautophagy; Mitophagy; PE; PET.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures
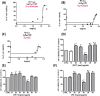

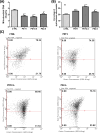


References
-
- Geyer R. Chapter 2 - Production, use, and fate of synthetic polymers. In: Plastic waste and recycling. environmental impact, societal issues, prevention, and solutions. 2020. p. 13–32. 10.1016/B978-0-12-817880-5.00002-5.
-
- Oliveri Conti G, Rapisarda P, Ferrante M. Relationship between climate change and environmental microplastics: a one health vision for the platysphere health. One Health Adv. 2024;2:17. 10.1186/s44280-024-00049-9.
-
- Garrido Gamarro E, Costanzo V. Microplastics in food commodities – a food safety review on human exposure through dietary sources. Food Safety and Quality Series No. 18. Rome: FAO; 2022. 10.4060/cc2392en.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

