Effect of focused ultrasound neuromodulation of the superior mesenteric plexus on insulin sensitivity and post-operative hyperglycemia in a swine model of surgical stress
- PMID: 40528253
- PMCID: PMC12175319
- DOI: 10.1186/s42234-025-00176-7
Effect of focused ultrasound neuromodulation of the superior mesenteric plexus on insulin sensitivity and post-operative hyperglycemia in a swine model of surgical stress
Abstract
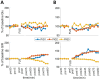
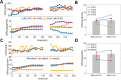
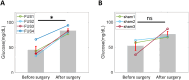
Metabolic stress during major surgery increases insulin resistance and causes post-operative hyperglycemia (POHG), which may in turn contribute to post-operative morbidity and mortality. Intensive insulin therapy for POHG is often ineffective and may even worsen patient outcomes. Non-invasive focused ultrasound stimulation (FUS) of glucose-sensing abdominal neurons improves glucose metabolism in animal models of diabetes, but its potential role in treating POHG remains unknown. In this study, we explored whether FUS of the superior mesenteric plexus (SMP) alters insulin sensitivity and post-operative fasting blood glucose (FBG) in a swine model of surgical stress-induced POHG. In each of 3 anesthetized animals, FUS targeting the porta hepatis (PH) of the liver or the SMP was delivered and insulin sensitivity was assessed in each case. In another series of experiments, 4 animals received SMP-FUS and 3 sham stimulation, after which surgical stress was induced via small bowel resection. In the 7 surgically operated animals, insulin sensitivity was measured before and after SMP-FUS (or sham), and fasting blood glucose (FBG) was measured before and 16 h after surgery. In all animals, insulin sensitivity was assessed using the hyperinsulinemic-euglycemic clamp (HEC) method. Results: SMP-FUS elicits a greater increase in insulin sensitivity than PH-FUS. On the day of surgery, SMP-FUS increases insulin sensitivity, compared to sham treatment. The day after surgery, surgically operated animals develop mild hyperglycemia. SMP-FUS-treated animals have higher FBG than sham-FUS-treated animals. No clear relationship is observed between FUS-induced changes in insulin sensitivity and next-day FBG. Conclusion: While SMP-FUS improves insulin sensitivity during surgery, it may exacerbate POHG.
Keywords: Focused ultrasound stimulation; Neuromodulation; Post-operative hyperglycemia; Superior mesenteric plexus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments were approved by the IACUC of the Feinstein Institute. Consent for publication: All authors consent for publication of this study. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Return to Running After Achilles Tendon Repair: How Do US Navy Service Members' Physical Readiness Tests Change After Undergoing an Achilles Tendon Repair?Clin Orthop Relat Res. 2025 Jun 18. doi: 10.1097/CORR.0000000000003590. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40536551
-
Mild cognitive impairment is not predictive of dementia up to 15 years after subthalamic deep brain stimulation in Parkinson's disease.J Parkinsons Dis. 2025 Jun;15(4):879-891. doi: 10.1177/1877718X251334049. Epub 2025 May 20. J Parkinsons Dis. 2025. PMID: 40390641
-
Use of β-adrenoreceptor drugs and Parkinson's disease incidence in women from the French E3N cohort study.J Parkinsons Dis. 2025 Jun;15(4):789-804. doi: 10.1177/1877718X251330993. Epub 2025 Apr 29. J Parkinsons Dis. 2025. PMID: 40302366
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Ashe J, Graf J, Madhavan R, Wallace K, Cotero V, Abate S, Pandey RK, Herzog R, Porindla SN, Shoudy D, Fan Y, Kao TJ, Puleo C. (2023). Investigation of liver-targeted peripheral focused ultrasound stimulation (pFUS) and its effect on glucose homeostasis and insulin resistance in type 2 diabetes mellitus: a proof of concept, phase 1 trial. QJM 116(8): 667–685. - PubMed
-
- Chang YC, Cracchiolo M, Ahmed U, Mughrabi I, Gabalski A, Daytz A, Rieth L, Becker L, Datta-Chaudhuri T, Al-Abed Y, Zanos TP, Zanos S. Quantitative Estimation of nerve fiber engagement by vagus nerve stimulation using physiological markers. Brain Stimul. 2020;13(6):1617–30. - PubMed
-
- Cotero V, Fan Y, Tsaava T, Kressel AM, Hancu I, Fitzgerald P, Wallace K, Kaanumalle S, Graf J, Rigby W, Kao TJ, Roberts J, Bhushan C, Joel S, Coleman TR, Zanos S, Tracey KJ, Ashe J, Chavan SS, Puleo C. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat Commun. 2019;10(1):952. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
