The Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) and its contributions to understanding Alzheimer's disease in Down syndrome: A decade of discovery
- PMID: 40528282
- PMCID: PMC12173842
- DOI: 10.1002/alz.70259
The Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) and its contributions to understanding Alzheimer's disease in Down syndrome: A decade of discovery
Abstract
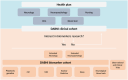
Down syndrome (DS) is a genetic form of Alzheimer's disease (AD) that offers crucial insights into AD pathogenesis. The Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) is a population-based multimodal biomarker cohort studying AD's natural history and clinical trials in DS. DABNI included 1135 participants (mean age 42.82, 46.3% female). At baseline, 673 participants were cognitively stable, 113 had prodromal AD, 239 had AD dementia, and 110 were uncertain due to non-AD conditions. Over 10 years, > 10000 clinical visits were conducted; follow-up showed that progression to symptomatic AD before age 40 was rare, but rates increased after age 50 (> 50% within 5 years). Neuropsychological and biomarker assessments demonstrated excellent diagnostic performance and a predictable sequence of changes, similar to autosomal dominant AD. DABNI participates in AD clinical trials and produced approximately 100 publications. The 10-year DABNI study provided critical insights into DS-associated AD (DSAD) and serves as a key platform for DS clinical trials. HIGHLIGHTS: Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) is a population-based multimodal cohort studying Alzheimer's disease in Down syndrome. Over 10 years, 1135 participants contributed to more than 10000 clinical visits and extensive biomarker studies. DABNI findings have transformed the understanding of Alzheimer's disease in Down syndrome, reinforcing its classification as a genetic form of the disease. The cohort integrates clinical care and research, enhancing early detection and patient management. DABNI supports clinical trials and has produced over 100 publications advancing Down syndrome-related Alzheimer's research.
Keywords: Alzheimer's disease; DABNI; Down syndrome; autosomal dominant Alzheimer's disease; biomarkers; clinical trials; cognition; health.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Juan Fortea reported receiving personal fees for service on the advisory boards, adjudication committees or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Novo Nordisk, Perha, Roche, Zambón and outside the submitted work. Daniel Alcolea, Alberto Lleó, and Juan Fortea reports holding a patent for markers of synaptopathy in neurodegenerative disease (licensed to ADx, EPI8382175.0). Sandra Giménez reported receiving personal fees for service on the advisory boards, speaker honoraria or educational activities from Esteve, Indorsia and Biojen. María Carmona‐Iragui reported receiving personal fees for service on the advisory boards, speaker honoraria or educational activities from Esteve, Lilly, Neuraxpharm, Adium, and Roche. JA reported receiving personal fees for service on the speaker honoraria or educational activities from Esteve, Lilly and Roche, outside the submitted work. DA reported receiving personal fees for advisory board services and/or speaker honoraria from Fujirebio‐Europe, Roche, Nutricia, Krka Farmacéutica, Lilly, Zambon S. A. U., Grifols, and Esteve, outside the submitted work. AL has served as a consultant or on advisory boards for Almirall, Fujirebio‐Europe, Roche, Biogen, Grifols, Novartis, Eisai, Lilly, and Nutricia, outside the submitted work. Valle Camacho reported receiving personal fees for service on the advisory boards, speaker honoraria or educational activities from General Electric, Life Molecular Imaging, Lilly and Novartis. Mateus Rozalem Aranha has provided paid consultancy for Veranex. Mateus Rozalem Aranha is a partner and director of production at Masima—Soluções em Imagens Médicas LTDA. María Carmona‐Iragui reported receiving personal fees for service on the advisory boards, speaker honoraria or educational activities from Esteve, Lilly, Neuraxpharm, Adium and Roche. Isabel Barroeta reported receiving personal fees for speaker honoraria from Adium. No other competing interests were reported. Author disclosures are available in the supporting information.
Figures


References
-
- Marilyn JB. Down syndrome. N Engl J Med. 2020;382(24):2344‐2352. - PubMed
-
- Ballard C, Williams G, Corbett A, Williams M, Hardy J. Dementia in down's syndrome. Lancet Neurol. 2016;15(6):622‐658. - PubMed
-
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17(3):278‐282. - PubMed
-
- Margallo‐Lana ML, Moore PB, Kay DWK, et al. Fifteen‐year follow‐up of 92 hospitalized adults with down's syndrome: incidence of cognitive decline, its relationship to age and neuropathology. J Intellect Disabil Res. 2007;51(pt 6):463‐477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- INT21/00073/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI20/01473/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI23/01786/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI20/00836/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI18/00335/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI22/00758/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- ICI23/00032/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI18/00435/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI22/00611/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- INT19/00016/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- INT23/00048/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI14/1561/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI20/01330/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI23/01767/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI22/00307/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI21/00791/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- PI24/00598/Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Gobierno de España)
- Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, Una Manera de Hacer Europa
- R01 AG056850/NH/NIH HHS/United States
- R21 AG056974/NH/NIH HHS/United States
- R01 AG061566/NH/NIH HHS/United States
- R01 AG081394/NH/NIH HHS/United States
- R61AG066543/NH/NIH HHS/United States
- 1RF1AG080769-01/NH/NIH HHS/United States
- GBHI_ALZ-18-543740/Global Brain Health Institute
- GBHI_ALZ-23-971107/Global Brain Health Institute
- IIBSP-DOW-2020-151/Fundación Tatiana Pérez de Guzmán el Bueno
- PCN00180/Fundación Tatiana Pérez de Guzmán el Bueno
- CD23/00235/Instituto de Salud Carlos III through the Sara Borrell Postdoctoral Fellowship
- CD20/00133/Instituto de Salud Carlos III through the Sara Borrell Postdoctoral Fellowship
- CM22/00052/Instituto de Salud Carlos III through the Río Hortega Fellowship
- CM21/00243/Instituto de Salud Carlos III through the Río Hortega Fellowship
- CM22/00219/Instituto de Salud Carlos III through the Río Hortega Fellowship
- CM22/00291/Instituto de Salud Carlos III through the Río Hortega Fellowship
- CP20/00038/Instituto de Salud Carlos III through the Miguel Servet
- CP24/00112/Instituto de Salud Carlos III through the Miguel Servet
- Miguel Servet program
- 23S06157-001/La Caixa Foundation and Ajuntament de Barcelona
- H2020-SC1-BHC-2018-2020/H2020 European Institute of Innovation and Technology
- PDC-2023-51/Fondation Jérôme Lejeune
- #1801Cycle2020/Fondation Jérôme Lejeune
- #1913cycle2019B/Fondation Jérôme Lejeune
- #2326-GRT-2024A/Fondation Jérôme Lejeune
- AARFD-21-852492/ALZ/Alzheimer's Association/United States
- AACSF-21-850193/ALZ/Alzheimer's Association/United States
- AARF-22-924456/ALZ/Alzheimer's Association/United States
- AARG-22-923680/ALZ/Alzheimer's Association/United States
LinkOut - more resources
Full Text Sources
Medical

