Current advances and unmet needs in Alzheimer's disease trials for individuals with Down syndrome: Navigating new therapeutic frontiers
- PMID: 40528298
- PMCID: PMC12173829
- DOI: 10.1002/alz.70258
Current advances and unmet needs in Alzheimer's disease trials for individuals with Down syndrome: Navigating new therapeutic frontiers
Abstract
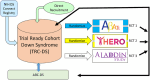
Individuals with Down syndrome (DS) have a genetically determined form of Alzheimer's disease (AD) due to the amyloid precursor protein (APP) gene dose effect. Nearly all individuals with DS develop AD pathology by age 40. Although dementia is rare before this age, its incidence rises sharply thereafter. Longitudinal studies estimate a lifetime dementia risk exceeding 90%, with prevalence reaching 88%-100% after age 65-a marked contrast to 10%-15% in the general population. Recent breakthroughs in sporadic AD, including anti-amyloid therapies such as lecanemab and donanemab, have shown efficacy in slowing progression. However, individuals with DS were excluded from these trials, leaving critical gaps in safety and efficacy data. This perspective highlights the current state of AD clinical trials in DS, key challenges-(including ethical considerations, recruitment barriers, and cognitive assessment adaptations), and emerging research efforts. Addressing these gaps is essential to ensure equitable access to disease-modifying therapies for individuals with DS. HIGHLIGHTS: Despite recent progress in Alzheimer's disease (AD) treatments for the general population-particularly monoclonal anti-amyloid therapies such as lecanemab and donanemab-individuals with Down syndrome (DS) were excluded from pivotal trials, leaving significant gaps in knowledge regarding safety and efficacy. A key concern in DS is the heightened risk of amyloid-related imaging abnormalities (ARIAs), a known side effect of anti-amyloid therapies, which may be aggravated by the increased prevalence and severity of cerebral amyloid angiopathy (CAA) in this population. For the first time, growing awareness of the nearly universal AD risk in DS is driving a stronger focus on tailored clinical research. Ongoing and forthcoming studies, including TRC-DS, ABATE, HERO, ALADDIN, and LESS-AD, are beginning to address these gaps. Beyond amyloid-targeting therapies, investigating alternative mechanisms such as tau pathology, neuroinflammation, and synaptic dysfunction is key to advancing treatments for DS-related AD. Collaboration between advocacy groups, researchers, and pharmaceutical companies is essential for overcoming barriers in AD clinical trials for DS, including ethical concerns, recruitment challenges, and the need for adapted cognitive assessments. This perspective also proposes strategies to enhance inclusivity in future studies, ensuring broader access to emerging treatments.
Keywords: Alzheimer's disease; Down syndrome; biomarkers; clinical trials; monoclonal antibodies.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
I.B. reported receiving personal fees for educational activities from Adium. L.V. has no disclosures. M.C.I. reported receiving personal fees for service on the advisory boards, speaker honoraria, or educational activities from Esteve, Lilly, Neuraxpharm, Adium, and Roche. J.F. reported receiving personal fees for service on the advisory boards and adjudication committees or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Novo Nordisk, Perha, Roche, and Zambón, outside the submitted work; and for holding a patent for markers of synaptopathy in neurodegenerative disease (licensed to ADx, EPI8382175.0). M.S.R. is a consultant to AC Immune and Ionis. He serves as Data Safety Monitoring Board Chair for Alzheon and Biohaven and is on the Scientific Advisory Board for Embic, Prescient, and Positrigo. His institution has received grants from Eisai and Lilly. Author disclosures are available in Supporting Information.
Figures
Similar articles
-
Stem cell-based therapeutic strategies for down syndrome and Alzheimer's disease.Stem Cell Res Ther. 2025 Aug 5;16(1):420. doi: 10.1186/s13287-025-04556-3. Stem Cell Res Ther. 2025. PMID: 40760500 Free PMC article. Review.
-
A meta-analysis and systematic review of interventions to prevent or treat cognitive decline related to Alzheimer's disease in adults with Down syndrome.Alzheimers Dement. 2025 Jul;21(7):e70471. doi: 10.1002/alz.70471. Alzheimers Dement. 2025. PMID: 40709486 Free PMC article. Review.
-
Amyloid-β peptide signature associated with cerebral amyloid angiopathy in familial Alzheimer's disease with APPdup and Down syndrome.Acta Neuropathol. 2024 Jul 18;148(1):8. doi: 10.1007/s00401-024-02756-4. Acta Neuropathol. 2024. PMID: 39026031 Free PMC article.
-
A 2025 update on treatment strategies for the Alzheimer's disease spectrum.J Chin Med Assoc. 2025 Jul 1;88(7):495-502. doi: 10.1097/JCMA.0000000000001252. Epub 2025 May 30. J Chin Med Assoc. 2025. PMID: 40442885 Review.
-
Joint spatial associations of amyloid beta and tau pathology in Down syndrome and preclinical Alzheimer's disease: Cross-sectional associations with early cognitive impairments.Alzheimers Dement. 2025 Jul;21(7):e70424. doi: 10.1002/alz.70424. Alzheimers Dement. 2025. PMID: 40588723 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

