Molecular mechanisms of high levels of L-ascorbic acid accumulation in chestnut rose fruits
- PMID: 40528345
- PMCID: PMC12365842
- DOI: 10.1016/j.xplc.2025.101419
Molecular mechanisms of high levels of L-ascorbic acid accumulation in chestnut rose fruits
Abstract
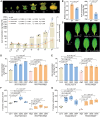
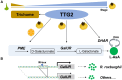
The fruit of chestnut rose (Rosa roxburghii Tratt.) contains exceptionally high levels of L-ascorbic acid (AsA) (∼1762 mg/100 g fresh weight), approximately 40-fold higher than those found in sweet orange (Citrus sinensis), which is well known for its high AsA content. However, the molecular mechanisms driving such high accumulation in chestnut rose remain unclear. Here, we report that the genes R. roxburghiiPECTIN METHYLESTERASE (RroxPME), D-GALACTURONATE REDUCTASE (RroxGalUR), and DEHYDROASCORBATE REDUCTASE 2 (RroxDHAR2) play crucial roles in AsA accumulation in chestnut rose fruit. By comparing R. roxburghii with the closely related Rosamultiflora, which has low AsA concentrations, we identified a 545-bp insertion in the promoter of RroxGalUR. We found that TRANSPARENT TESTA GLABRA 2 (RroxTTG2), a well-known key regulator of trichome development, binds to the W-box-containing inserted region of the RroxGalUR promoter as well as the promoters of RroxPME and RroxDHAR2. In contrast, in sweet orange, CsTTG2 can bind only to CsPME. Furthermore, RroxTTG2 retains its conserved role in the regulation of trichome development during early fruit development, suggesting its spatiotemporal specificity in regulating both trichome development and AsA biosynthesis. To evaluate the application value of this pathway in other species, we heterologously expressed RroxTTG2, RroxPME, RroxGalUR, and RroxDHAR2 in lettuce (Lactuca sativa L.), which increased AsA concentrations in the transgenic lines by up to 355% (an increase from approximately 2 to 10 mg/100 g fresh weight). This study provides insights into mechanisms underlying AsA accumulation in chestnut rose and the spatiotemporal transcriptional regulation of AsA biosynthesis and trichome development.
Keywords: L-ascorbic acid; Rosa roxburghii; fruit; transcriptional regulation; trichomes.
Copyright © 2025. Published by Elsevier Inc.
Figures




References
-
- Agius F., González-Lamothe R., Caballero J.L., Muñoz-Blanco J., Botella M.A., Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003;21:177–181. - PubMed
-
- Berhin A., Nawrath C., Hachez C. Subtle interplay between trichome development and cuticle formation in plants. New Phytol. 2022;233:2036–2046. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

