Discrepancies in assessing intellectual disability levels in adults with Down syndrome: Implications for dementia diagnosis
- PMID: 40528393
- PMCID: PMC12173952
- DOI: 10.1002/alz.70307
Discrepancies in assessing intellectual disability levels in adults with Down syndrome: Implications for dementia diagnosis
Abstract
Introduction: Cut-offs derived from baseline cognitive assessments, stratified by intellectual disability (ID) level, have been proposed to diagnose symptomatic Alzheimer's disease (AD) in Down syndrome (DS). However, discrepancies in ID classification risk misclassification when applying cut-offs across sites.
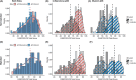
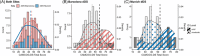
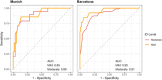
Methods: This dual-center cohort study included 673 adults with mild to moderate ID at different AD stages. We assessed ID classification discrepancies across sites and the impact on Cambridge Cognitive Examination for Older Adults with Down's Syndrome (CAMCOG-DS) cut-offs for AD dementia diagnosis derived from receiver operating characteristic analysis.
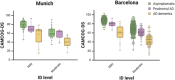
Results: Inter-rater agreement for ID level classification was 95% within sites but 60% between sites. While CAMCOG-DS score distributions in the whole cohort were similar across sites, ID classification discrepancies caused higher cut-offs in Barcelona for mild and moderate ID compared to Munich. Applying site-specific cut-offs to another cohort reduced sensitivity and specificity.
Discussion: Standardizing ID classification is critical for generalizable cut-offs to accurately diagnose AD dementia based on neuropsychological assessments in DS.
Highlights: CAMCOG-DS cut-offs by intellectual disability level classify dementia in Down syndrome. ID classification discrepancies between sites impact CAMCOG-DS diagnostic cut-offs. Applying site-specific cut-offs to other cohorts reduces sensitivity and specificity. Standardized ID classification is essential for generalizable cognitive cut-offs. Use site-specific cut-offs until ID classification is standardized.
Keywords: AD21; Alzheimer's disease; Cambridge Cognitive Examination for Older Adults with Down Syndrome; Down Alzheimer Barcelona Neuroimaging Initiative; Down syndrome; Down syndrome–associated Alzheimer's disease; cut‐off points; dementia; diagnostic performance; intellectual disability.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
J.F. has received personal fees for service on advisory boards, adjudication committees, or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Perha, and Roche, outside the submitted work. J.F. also holds a patent for markers of synaptopathy in neurodegenerative disease (licensed to Adx, EPI8382175.0). J.L. reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA, Zambon, Esteve, Merck, and Roche; consulting fees from Axon Neuroscience, EISAI, and Biogen; author fees from Thieme Medical Publishers and W. Kohlhammer GmbH medical publishers; and is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4‐Repeat Tauopathies” (PCT/EP2024/053388) filed by LMU Munich. In addition, J.L. reports compensation for serving as chief medical officer for MODAG GmbH, is beneficiary of the phantom share program of MODAG GmbH, and is inventor in a patent “Pharmaceutical Composition and Methods of Use” (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work. The other authors report no relevant disclosures. Author disclosures are available in the supporting information.
Figures
Similar articles
-
The Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) and its contributions to understanding Alzheimer's disease in Down syndrome: A decade of discovery.Alzheimers Dement. 2025 Jun;21(6):e70259. doi: 10.1002/alz.70259. Alzheimers Dement. 2025. PMID: 40528282 Free PMC article. Review.
-
Measurement properties of the German version of the Cambridge examination for mental disorders of older people with Down syndrome and others with intellectual disabilities (CAMDEX-DS).J Intellect Dev Disabil. 2024 Dec;49(4):402-414. doi: 10.3109/13668250.2024.2317794. Epub 2024 Feb 29. J Intellect Dev Disabil. 2024. PMID: 39815946
-
Validation of the CAMCOG-DS-II, a neuropsychological test battery for Alzheimer's disease in people with Down syndrome: A Horizon 21 European Down syndrome Consortium study.Alzheimers Dement. 2025 Mar;21(3):e70071. doi: 10.1002/alz.70071. Alzheimers Dement. 2025. PMID: 40145416 Free PMC article.
-
Timing of Alzheimer's Disease by Intellectual Disability Level in Down Syndrome.J Alzheimers Dis. 2023;95(1):213-225. doi: 10.3233/JAD-230200. J Alzheimers Dis. 2023. PMID: 37482997 Free PMC article.
-
Current advances and unmet needs in Alzheimer's disease trials for individuals with Down syndrome: Navigating new therapeutic frontiers.Alzheimers Dement. 2025 Jun;21(6):e70258. doi: 10.1002/alz.70258. Alzheimers Dement. 2025. PMID: 40528298 Free PMC article. Review.
Cited by
-
The Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) and its contributions to understanding Alzheimer's disease in Down syndrome: A decade of discovery.Alzheimers Dement. 2025 Jun;21(6):e70259. doi: 10.1002/alz.70259. Alzheimers Dement. 2025. PMID: 40528282 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- Program1/Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas
- La Caixa Foundation
- AARG-22-923680 to A.B/ALZ/Alzheimer's Association/United States
- AARG-22-973966 to M.C-I/ALZ/Alzheimer's Association/United States
- INT21/00073/Carlos III Health Institute
- CM23/00291/Carlos III Health Institute
- PI20/01473/Carlos III Health Institute
- PI23/01786 to J.F/Carlos III Health Institute
- PI22/00785 to M.C-I/Carlos III Health Institute
- PI22/00307 to AB/Carlos III Health Institute
- CP24/00112 to L.D.H.S/Carlos III Health Institute and European Union, Miguel Servet Program
- CP20/00038 to A.B/Carlos III Health Institute and European Union, Miguel Servet Program
- CD23/00235 to L.V/Carlos III Health Institute, Sara Borrell
- CM22/00219 to J.E.A.-I/Carlos III Health Institute, Sara Borrell
- CM22/00052 to I.R.-B/Carlos III Health Institute, Sara Borrell
- CM23/00291 to L.M.B/Carlos III Health Institute, Sara Borrell
- CM22/00219 to J.E.A.I./Carlos III Health Institute and European Union Río-Hortega program
- CM22/00052 to I.R.B./Carlos III Health Institute and European Union Río-Hortega program
- CM23/00291 to L.M.B/Carlos III Health Institute and European Union Río-Hortega program
- U01AG024904/NH/NIH HHS/United States
- R01AG056850/NH/NIH HHS/United States
- R21AG056974/NH/NIH HHS/United States
- R01AG061566/NH/NIH HHS/United States
- R01AG081394/NH/NIH HHS/United States
- R61AG066543 to J.F/NH/NIH HHS/United States
- LT006/17/00119 to J.F/Department de Salut de la Generalitat de Catalunya Pla Estrategic de Recerca I Innovació en Salut
- IIBSP-DOW-2020-151 to J.F/Fundación Tatiana Pérez de Guzmán el Bueno
- H2020-SC1-BHC-2018-2020 to J.F/Research and Innovation Framework Program
- 2326 - GRT-2024A to L.D.H.S/Jérôme Lejeune Foundation
- AARG-22-923680 to A.B/Jérôme Lejeune Foundation
- AARG-22-973966 to M.C-I/Jérôme Lejeune Foundation
- VERUM Foundation
- EXC2145SyNergy-ID390857198/Deutsche Forschungsgemeinschaft DFG, German Research Foundation, under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology
- T21RS Scientific Exchange Grant to K.S
- SLT006/17/00119/Departament de Salut, Generalitat de Catalunya
LinkOut - more resources
Full Text Sources
Medical

