Chorionic Villus Sampling for Rapid Confirmation of High-Risk NIPT Results for Trisomy 21, 18, and 13
- PMID: 40528522
- PMCID: PMC12254428
- DOI: 10.1002/pd.6837
Chorionic Villus Sampling for Rapid Confirmation of High-Risk NIPT Results for Trisomy 21, 18, and 13
Abstract
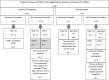
Objectives: International societies recommend amniocentesis (AC) after high-risk non-invasive prenatal testing (NIPT) because of potential inconclusive results from chorionic villus sampling (CVS) caused by placental mosaicism. Our study aimed to evaluate the necessity of confirmatory amniocentesis following CVS for trisomies 21, 18, and 13 with separate analysis of cytotrophoblast (CTB) and mesenchymal core (MC).
Methods: We retrospectively analyzed the confirmatory cytogenetic results between April 2017 and December 2022. CTB and MC were separated and analyzed by QF-PCR and/or SNP array, and karyotyping when needed.
Results: Among 338 cases, 70% (237/339) of women underwent CVS (70.5%) and 30% (101/338) underwent AC. Mosaic trisomy in MC requiring additional amniocentesis was detected in 13.5% (5/37) of cases referred due to trisomy 13, 2.5% (4/158) of cases of trisomy 21% and 0% (0/42) of cases of trisomy 18.
Conclusions: A definitive diagnosis of CVS was achieved in 97.5%, 100%, and 86.5% of patients with high-risk NIPT results for trisomy 21, 18, and 13, respectively. Moreover, our clinical practice confirms that the majority of pregnant women (70%) opted for CVS as a quick confirmatory test. We conclude that both CVS and AC can be offered when preceded by pre-test counseling on the risks of potential inconclusive results as calculated in this study.
Keywords: CVS; NIPT; amniocentesis; chorionic villi sampling; follow‐up test; non‐invasive prenatal test; prenatal diagnosis.
© 2025 The Author(s). Prenatal Diagnosis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Van Opstal D., Srebniak M. I., Polak J., et al., “False Negative NIPT Results: Risk Figures for Chromosomes 13, 18 and 21 Based on Chorionic Villi Results in 5967 Cases and Literature Review,” PLoS One 11, no. 1 (2016): e0146794, [Electronic Resource], 10.1371/journal.pone.0146794. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

