Impact of Fiber Orientation in Electrospun PLA Scaffolds on Fluid Dynamics in a Custom Microfluidic Device
- PMID: 40528562
- PMCID: PMC12477572
- DOI: 10.1002/adhm.202500378
Impact of Fiber Orientation in Electrospun PLA Scaffolds on Fluid Dynamics in a Custom Microfluidic Device
Abstract
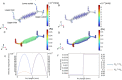
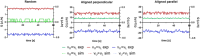
Organ-on-Chip (OoC) systems are evolving as vital tools in biomedical research, proposing advanced platforms to replicate human tissue microenvironments for drug testing and disease modeling. This study examines how the orientation of polylactic acid (PLA) fibers influences fluid movement in a custom OoC setup. PLA scaffolds are fabricated via electrospinning with either random or aligned fiber orientations. Scanning electron microscopy (SEM) reveals that random scaffolds are 70 µm thick with fibers measuring 1.12 µm, while aligned scaffolds are thinner at 35 µm with fibers of 1.02 µm. Porosity and matrix structure are analyzed to understand the impact of fiber arrangement. Liquid water permeability is tested using a custom three dimensional (3D)-printed device conforming to ISO 7198:2016 standards. Computational fluid dynamics (CFD) simulations, employing the Porous Media Flow Module and Brinkman's equations, predict flow behavior based on scaffold morphology. A dual-chamber microfluidic chip integrated with pressure sensors allows real-time measurements to validate the simulations. Results demonstrate that fiber alignment significantly alters scaffold permeability and flow dynamics. These insights are valuable for tissue engineering, offering a validated framework to design microfluidic devices with tailored fluidic environments optimized for specific scaffold architectures.
Keywords: Organ‐on‐Chip; computational fluid dynamics (CFD); electrospinning; fiber alignment; liquid permeability; microfluidic devices.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Ong L. J. Y., Sun A. R., Wang Z., Lee J., Prasadam I., Toh Y. C., Adv. Funct. Mater. 2024, 34, 2315608.
-
- Cartmell S., Porter B., García A., Guldberg R., Tissue Eng. 2003, 9, 1197. - PubMed
-
- Ahmed T., Biosens Bioelectron X 2022, 11, 100194.
-
- Zhang Y. E., Pappas D., Mechref Y., Shi H., Thompson J. E., Sheridan M., 2017.
MeSH terms
Substances
Grants and funding
- Sviluppo di sistemi cellulari tridimensionali integrati con approcci invivitrosi e tecniche microfluidiche per lo sviluppo di modelli sostitutivi del modello animale (PJ_MIN_SALUTE_METODI_SOSTITUTIVI_2022)/Ministero della Salute
- ECS00000022/MUR-PNRR project Ecosistema dell'Innovazione Sicilian MicronanoTech Research And Innovation Center (SAMOTHRACE)
- Spoke3-WP5-T5.2.1./MUR-PNRR project Ecosistema dell'Innovazione Sicilian MicronanoTech Research And Innovation Center (SAMOTHRACE)
LinkOut - more resources
Full Text Sources
Miscellaneous

