Deadly Decomposers: Distinguishing Life History Strategies on the Parasitism-Saprotrophy Spectrum
- PMID: 40528811
- PMCID: PMC12175056
- DOI: 10.1111/ele.70135
Deadly Decomposers: Distinguishing Life History Strategies on the Parasitism-Saprotrophy Spectrum
Abstract
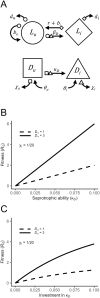
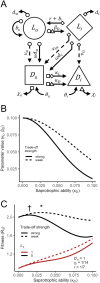
The ability to parasitize living hosts as well as decompose dead organic matter is both common and widespread across prokaryotic and eukaryotic taxa. These parasitic decomposers have long been considered merely accidental or facultative parasites. However, this is often untrue: in many cases, parasitism is integral to the ecology and evolution of these organisms. Combining life cycle information from the literature with a generalised eco-evolutionary model, we define four distinct life history strategies followed by parasitic decomposers. Each strategy has a unique fitness expression, life cycle, ecological context, and set of evolutionary constraints. Correctly classifying parasitic decomposers is essential for understanding their ecology and epidemiology and directly impacts efforts to manage important medical and agricultural pathogens.
Keywords: biotroph‐necrotroph spectrum; entomopathogens; evolution of parasitism; facultative parasites; life history; nosocomial diseases; opportunistic pathogens; sapronosis; trade‐offs.
© 2025 The Author(s). Ecology Letters published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Abang, M. M. , Baum M., Ceccarelli S., et al. 2006. “Differential Selection on Rhynchosporium Secalis During Parasitic and Saprophytic Phases in the Barley Scald Disease Cycle.” Phytopathology 96: 1214–1222. - PubMed
-
- Akinsanmi, O. A. , Chakraborty S., Backhouse D., and Simpfendorfer S.. 2007. “Passage Through Alternative Hosts Changes the Fitness of Fusarium Graminearum and Fusarium Pseudograminearum.” Environmental Microbiology 9: 512–520. - PubMed
-
- Andersen, S. B. , Gerritsma S., Yusah K. M., et al. 2009. “The Life of a Dead Ant: The Expression of an Adaptive Extended Phenotype.” American Naturalist 174: 424–433. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

