Thermosensitive hydrogel composite with si-Cx43 nanoparticles and anti-VEGF agent for synergistic treatment of diabetic retinopathy
- PMID: 40528841
- PMCID: PMC12173670
- DOI: 10.1016/j.mtbio.2025.101917
Thermosensitive hydrogel composite with si-Cx43 nanoparticles and anti-VEGF agent for synergistic treatment of diabetic retinopathy
Abstract
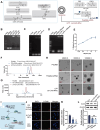
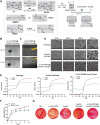
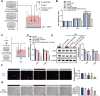
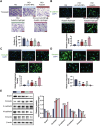
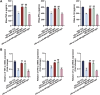
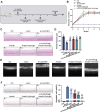
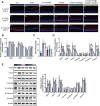
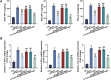
Diabetic retinopathy (DR) is characterized by pathological angiogenesis, inflammation, and retinal neurodegeneration, leading to vision loss. Current therapies, such as anti-VEGF agents, face challenges of low bioavailability and frequent invasive injections. Connexin43 (Cx43), a gap junction protein, plays a key role in DR progression through its modulation of inflammation and vascular dysfunction. A thermosensitive hydrogel composite was developed to encapsulate siRNA targeting Cx43 (si-Cx43) nanoparticles (NPs) and anti-VEGF (Avastin). The hydrogel was characterized for gelation, injectability, and degradation. In vitro studies evaluated the cytotoxicity, anti-angiogenic effects, and permeability regulation in hyperglycemic retinal cells under hyperglycemic conditions. In vivo therapeutic efficacy was assessed in a diabetic retinopathy rat model. si-Cx43-NPs demonstrated high siRNA encapsulation efficiency and stability, effectively silencing Cx43 expression in retinal endothelial cells. The hydrogel exhibited excellent injectability, temperature-sensitive gelation, and controlled degradation. In vitro, si-Cx43-NPs@Avastin-hydrogel significantly suppressed VEGF expression, reduced angiogenesis, and restored cell permeability under hyperglycemic conditions. In vivo, the hydrogel composite reduced neovascularization, inflammation, and apoptosis, restoring retinal structure and function more effectively than either single-agent treatment alone. Biocompatibility studies confirmed minimal toxicity and favorable degradation. The si-Cx43-NPs@Avastin-hydrogel provides a synergistic and minimally invasive therapeutic strategy for DR by targeting angiogenesis, inflammation, and neuroprotection with sustained drug delivery.
Keywords: Angiogenesis; Anti-VEGF; Diabetic retinopathy; Inflammation; Thermosensitive hydrogel; si-Cx43 nanoparticles.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats.J Orthop Translat. 2025 Jun 5;53:63-81. doi: 10.1016/j.jot.2025.04.015. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40529900 Free PMC article.
-
Hyaluronic acid methacryloyl-based co-delivery system for aflibercept and miR-21-3p antagomir: a dual-therapeutic approach for diabetic retinopathy.J Control Release. 2025 Aug 3:114077. doi: 10.1016/j.jconrel.2025.114077. Online ahead of print. J Control Release. 2025. PMID: 40763825
-
Ghrelin alleviates high glucose-induced retinal microvascular endothelial cell injury by activating Nrf2/HO-1 pathway to inhibit ferroptosis.Int J Ophthalmol. 2025 Jun 18;18(6):978-985. doi: 10.18240/ijo.2025.06.02. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40534808 Free PMC article.
-
Efficacy and safety of Chinese botanical drug Si Shen Wan in irritable bowel syndrome: a meta-analysis and trial sequential analysis of randomized controlled trials.Front Pharmacol. 2025 Jun 2;16:1534904. doi: 10.3389/fphar.2025.1534904. eCollection 2025. Front Pharmacol. 2025. PMID: 40529486 Free PMC article.
-
Modulation of Oxidative Stress in Diabetic Retinopathy: Therapeutic Role of Natural Polyphenols.Antioxidants (Basel). 2025 Jul 17;14(7):875. doi: 10.3390/antiox14070875. Antioxidants (Basel). 2025. PMID: 40722979 Free PMC article. Review.
References
-
- Avogaro A., Fadini G.P. Microvascular complications in diabetes: a growing concern for cardiologists. Int. J. Cardiol. 2019;291:29–35. - PubMed
-
- Bourne R.R.A., Jonas J.B., Bron A.M., Cicinelli M.V., Das A., Flaxman S.R., Friedman D.S., Keeffe J.E., Kempen J.H., Leasher J., Limburg H., Naidoo K., Pesudovs K., Peto T., Saadine J., Silvester A.J., Tahhan N., Taylor H.R., Varma R., Wong T.Y., Resnikoff S. Vision loss expert group of the global burden of disease S. Prevalence and causes of vision loss in high-income countries and in Eastern and central Europe in 2015: magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018;102:575–585. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

