Engineered in-situ-forming biomimetic hydrogel with self-regulated immunostimulatory capacity promotes postoperative tumor treatment
- PMID: 40528972
- PMCID: PMC12167896
- DOI: 10.1016/j.fmre.2023.02.029
Engineered in-situ-forming biomimetic hydrogel with self-regulated immunostimulatory capacity promotes postoperative tumor treatment
Abstract
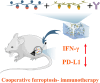
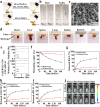
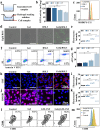
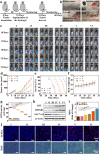
Post-resection tumors with microscopic foci and immunosuppressive microenvironments have high risk of recurrence and metastasis but respond poorly to various therapies. Herein, we propose a biomimetic hydrogel as a biocompatible, biodegradable and bioadhesive postoperative dressing that could be formed in situ by NaIO4-initiated thiourea-catechol crosslinking after syringe-injection into the resection cavity. The thiourea or catechol-bearing hyaluronic acid precursors are also separately engineered with phenylboronic acid and β-cyclodextrin (β-CD) groups, potentiating the reversible immobilization of (1S, 3R) RAS-selective lethal 3 (RSL3) and glycosylated granulocyte macrophage-colony stimulating factor (GM-CSF) without invasive chemical reactions. Meanwhile, the interconnected porous superstructure of the hydrogels allows the incorporation and self-regulated delivery of PD-L1 antibody (aPD-L1). RSL3-induced immunogenic ferroptosis and GM-CSF could cooperatively trigger robust adaptive tumor-specific immune responses, while aPD-L1 further alleviates the accumulated immunoresistance of tumor cells due to interferon γ-mediated PD-L1 upregulation, thus stimulating potent local and whole-body antitumor immunity to prevent postoperative tumor recurrence and metastasis. The biomimetic hydrogel may serve as a promising solution for the postoperative treatment of solid tumors.
Keywords: Cooperative ferroptosis-immunotherapy; Injectable in-situ forming hydrogel; Microenvironment remodeling; Postoperative tumor therapy; Supramolecular bioresponsive prodrug.
© 2023 The Authors. Publishing Services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures




Similar articles
-
Tumor-derived CD109 orchestrates reprogramming of tumor-associated macrophages to dampen immune response.J Hepatol. 2025 Apr 11:S0168-8278(25)00231-4. doi: 10.1016/j.jhep.2025.03.035. Online ahead of print. J Hepatol. 2025. PMID: 40220905
-
Mitigation and Management of Common Toxicities Associated with the Administration of CAR-T Therapies in Oncology Patients.Drug Saf. 2025 Jul;48(7):719-737. doi: 10.1007/s40264-025-01538-5. Epub 2025 Mar 19. Drug Saf. 2025. PMID: 40108072 Free PMC article. Review.
-
Use of β-adrenoreceptor drugs and Parkinson's disease incidence in women from the French E3N cohort study.J Parkinsons Dis. 2025 Jun;15(4):789-804. doi: 10.1177/1877718X251330993. Epub 2025 Apr 29. J Parkinsons Dis. 2025. PMID: 40302366
-
Injectable microenvironment-responsive hydrogels with redox-activatable supramolecular prodrugs mediate ferroptosis-immunotherapy for postoperative tumor treatment.Acta Biomater. 2023 Oct 1;169:289-305. doi: 10.1016/j.actbio.2023.08.002. Epub 2023 Aug 5. Acta Biomater. 2023. PMID: 37544392
-
Therapeutic potential of adenosine receptor modulators in cancer treatment.RSC Adv. 2025 Jun 17;15(26):20418-20445. doi: 10.1039/d5ra02235e. eCollection 2025 Jun 16. RSC Adv. 2025. PMID: 40530308 Free PMC article. Review.
References
-
- Waks A.G., Winer E.P. Breast cancer treatment a review. JAMA. 2019;321(3):288–300. - PubMed
-
- Hiller J.G., Perry N.J., Poulogiannis G., et al. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018;15(4):205–218. - PubMed
-
- Hosein A.N., Dougan S.K., Aguirre A.J., et al. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer. 2022;3(3):272–286. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

