Enhanced Gram-Negative Membrane Disruption and In Vivo Efficacy via Lysine-Arginine Enrichment of Opis16a
- PMID: 40529087
- PMCID: PMC12169475
- DOI: 10.1021/acsmedchemlett.5c00038
Enhanced Gram-Negative Membrane Disruption and In Vivo Efficacy via Lysine-Arginine Enrichment of Opis16a
Abstract
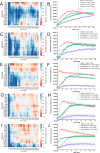
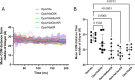
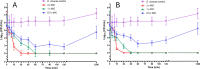
Infections complicate burn wound care, especially with the rise of antimicrobial resistance. Antimicrobial peptides (AMPs) offer the potential for advancing wound care by combating persistent infections. Opis16a, a scorpion venom-derived AMP, exhibits potent antibacterial activity by targeting Gram-negative membranes, causing rapid membrane disruption and bacterial cell death. Here, four novel Opis16a analogues were developed with improved membrane targeting and antibacterial efficacy. One analogue shows particular promise for topical application in Gram-negative burn wound infections. Enhanced peptide-lipid hydrogen bonding increases conformational stability, membrane insertion, and permeabilization rates. Substituting lysine residues in the C-terminal with arginine leads to the most consistent improvement in activity, selectivity for pathogen over HaCat cells, and stability in serum. In an in vivo Galleria mellonella burn wound model, a 5 mg/kg topical dose provides better protection than Opis16a against Enterobacter cloacae NICD 16103. These findings highlight the potential of optimized bactericidal AMPs to improve burn wound care.
Keywords: E. cloacae; Gram-negative bacteria; Opis16a; Opis16aCterKR; antimicrobial peptides; antimicrobial resistance; arginine substitution; membrane permeabilization; molecular dynamics; wound infections.
© 2025 The Authors. Published by American Chemical Society.
Figures




References
-
- Harris P. N., Wei J. Y., Shen A. W.. et al. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J. Antimicrob. Chemother. 2016;71(2):296–306. doi: 10.1093/jac/dkv346. - DOI - PubMed
LinkOut - more resources
Full Text Sources

