Upregulation of LINC02154 promotes esophageal cancer progression by enhancing cell cycling and epithelial-mesenchymal transition
- PMID: 40529228
- PMCID: PMC12173678
- DOI: 10.1016/j.ncrna.2025.06.001
Upregulation of LINC02154 promotes esophageal cancer progression by enhancing cell cycling and epithelial-mesenchymal transition
Abstract
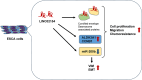
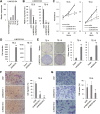
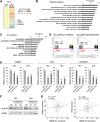
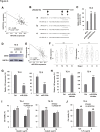
Long noncoding RNAs (lncRNAs) play crucial roles in the progression of human malignancies; however, their involvement in esophageal cancer (ESCA) remains incompletely understood. In this study, we screened for lncRNAs upregulated in ESCA and identified 12 lncRNAs significantly upregulated in primary ESCA tumors. Among those, elevated LINC02154 expression correlated positively with advanced T stages. LINC02154 knockdown in ESCA cell lines suppressed cell proliferation and migration, while ectopic expression of LINC02154 enhanced colony formation. Depletion of LINC02154 suppressed genes involved in various oncogenic processes, including cell cycling, epithelial-mesenchymal transition (EMT), and metabolism. We also found that LINC02154 promotes EMT and enhances chemoresistance, at least in part, through suppression of miR-200b. Finally, RNA-pulldown and mass spectrometry analysis revealed that LINC02154 interacts with proteins involved in the cornified envelope or desmosome. These findings suggest that LINC02154 exerts oncogenic effects through modulation of multiple oncogenic signaling pathways in ESCA and that LINC02154 is a potential therapeutic target.
Keywords: Cell cycle; ESCA; Esophageal cancer; VIM; lncRNA; miR-200b.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
LINC02154 promotes cell cycle and mitochondrial function in oral squamous cell carcinoma.Cancer Sci. 2025 Feb;116(2):393-405. doi: 10.1111/cas.16379. Epub 2024 Nov 22. Cancer Sci. 2025. PMID: 39576738 Free PMC article.
-
Targeting Drp1 inhibits ESCC progression via the ROS-PGC1-α-Nrf1/2 pathway.J Transl Med. 2025 Jun 17;23(1):674. doi: 10.1186/s12967-025-06697-8. J Transl Med. 2025. PMID: 40528181 Free PMC article.
-
The lncRNA MIR22HG suppresses prostate cancer cell proliferation, migration, and epithelial-mesenchymal transition via the miR-4428/PCDH9 axis.Transl Cancer Res. 2025 May 30;14(5):3133-3148. doi: 10.21037/tcr-2024-2200. Epub 2025 May 14. Transl Cancer Res. 2025. PMID: 40530115 Free PMC article.
-
Therapeutic potential of adenosine receptor modulators in cancer treatment.RSC Adv. 2025 Jun 17;15(26):20418-20445. doi: 10.1039/d5ra02235e. eCollection 2025 Jun 16. RSC Adv. 2025. PMID: 40530308 Free PMC article. Review.
-
Prevalence and odds of anxiety and depression in cutaneous malignant melanoma: a proportional meta-analysis and regression.Br J Dermatol. 2024 Jun 20;191(1):24-35. doi: 10.1093/bjd/ljae011. Br J Dermatol. 2024. PMID: 38197404
References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74:229–263. - PubMed
-
- Lagergren J., Smyth E., Cunningham D., Lagergren P. Oesophageal cancer. The Lancet. 2017;390:2383–2396. - PubMed
-
- Rustgi A.K., El-Serag H.B. Esophageal carcinoma. N. Engl. J. Med. 2014;371:2499–2509. - PubMed
-
- Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 2009;10:155–159. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

