Microbiota and gastric cancer: from molecular mechanisms to therapeutic strategies
- PMID: 40529304
- PMCID: PMC12170596
- DOI: 10.3389/fcimb.2025.1563061
Microbiota and gastric cancer: from molecular mechanisms to therapeutic strategies
Abstract
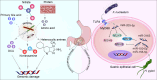
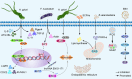
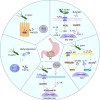
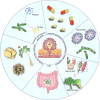
Gastric cancer, a prevalent malignancy globally, is influenced by various factors. The imbalance in the gut microbiome and the existence of particular intratumoural microbiota could have a strong connection with the onset and progression of gastric cancer. High-throughput sequencing technology and bioinformatics analysis have revealed a close correlation between abnormal abundance of specific microbial communities and the risk of gastric cancer. These microbial communities contribute to gastric cancer progression through mechanisms including increasing cellular genomic damage, inhibiting DNA repair, activating abnormal signaling pathways, exacerbating tumor hypoxia, and shaping a tumor immune-suppressive microenvironment. This significantly impacts the efficacy of gastric cancer treatments, including chemotherapy and immunotherapy. Probiotic, prebiotic, antibiotic, carrier-based, dietary interventions, fecal microbiota transplantation, and traditional Chinese medicine show potential applications in gastric cancer treatment. However, the molecular mechanisms regarding dysbiosis of microbiota, including gut microbiota, and intra-tumoral microbiota during the progression of gastric cancer, as well as the therapeutic efficacy of microbiota-related applications, still require extensive exploration through experiments.
Keywords: gastric cancer; microbiota; molecular mechanisms; targeted therapy; tumor microenvironment.
Copyright © 2025 Chen, Jin, Hu, Guan, Bai and Gou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Gut modulation to regulate NF-κB in colorectal and gastric cancer therapy and inflammation.Cancer Immunol Immunother. 2025 Jul 12;74(8):264. doi: 10.1007/s00262-025-04118-9. Cancer Immunol Immunother. 2025. PMID: 40650758 Free PMC article. Review.
-
Radiation-induced injury and the gut microbiota: insights from a microbial perspective.Therap Adv Gastroenterol. 2025 Jun 16;18:17562848251347347. doi: 10.1177/17562848251347347. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40535532 Free PMC article. Review.
-
Anxiety-like behavior during protracted morphine withdrawal is driven by gut microbial dysbiosis and attenuated with probiotic treatment.Gut Microbes. 2025 Dec;17(1):2517838. doi: 10.1080/19490976.2025.2517838. Epub 2025 Jun 15. Gut Microbes. 2025. PMID: 40518557 Free PMC article.
-
Gut microbiota implication in diabetic kidney disease: mechanisms and novel therapeutic strategies.Ren Fail. 2025 Dec;47(1):2517402. doi: 10.1080/0886022X.2025.2517402. Epub 2025 Jun 25. Ren Fail. 2025. PMID: 40563141 Review.
-
Microbial imbalance in the gut: a new frontier in Rheumatoid arthritis research.Inflammopharmacology. 2025 May;33(5):2277-2291. doi: 10.1007/s10787-025-01737-7. Epub 2025 Apr 12. Inflammopharmacology. 2025. PMID: 40220199 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

