A new era in the treatment of progressive fibrosing interstitial lung diseases
- PMID: 40529319
- PMCID: PMC12171857
- DOI: 10.1183/20734735.0259-2024
A new era in the treatment of progressive fibrosing interstitial lung diseases
Abstract
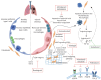
Idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) are characterised by an irreversible progression of pulmonary fibrosis and functional lung decline. Current antifibrotic therapies (nintedanib and pirfenidone for IPF and nintedanib for PPF) can reduce disease progression but not halt or reverse it. PPF and IPF share common pathophysiological pathways that need to be further elucidated for the development of novel therapeutic strategies. The educational aim of this review is to explain the pathogenic pathways that have led to the discovery of new therapeutic agents and their favourable implementation in phase 2 and 3 studies. This includes phosphodiesterase 4 inhibitors, αvβ6 and αvβ1 integrin inhibitors, lymphosphatidic acid antagonists, inhaled treprostinil, hedgehog inhibitors, tyrosine kinase inhibitors and angiotensin type 2 receptor agonists. The aim is also to better understand current therapeutic challenges and future perspectives, including cellular therapies, exosomes and their cargoes, as well as the integration of transcriptomics and proteomics, plus gene therapy.
Copyright ©ERS 2025.
Conflict of interest statement
Conflict of interest: A. Tzouvelekis has received advisory fees and travel grants from Boehringer Ingelheim, Hoffman La Roche, GSK, AstraZeneca, Menarini, Guidotti, Pliant, BMS, Pfizer, Gilead, Chiesi, Elpen, MannKind, Puretech and Medochemie, outside the submitted work. A. Tzouvelekis is the holder of two therapeutic patents: “Inhaled or aerosolized delivery of thyroid hormone and analogues to the lung as a novel therapeutic agent in fibrotic lung diseases” OCR#6368 disclosed to Yale University. J. Guiot reports personal fees for advisory board work and lectures from Boehringer Ingelheim, Janssen, SMB, GSK, Roche, AstraZeneca, Aquilon, Volition, Oncoradiomics and Chiesi, non-financial support for meeting attendance from AstraZeneca, Chiesi, MSD, Roche, Boehringer Ingelheim and Janssen. J. Guiot is on the permanent SAB of Radiomics (Oncoradiomics SA) for the SALMON trial without any specific consultancy fee for this work. J. Guiot is co-inventor of one issued patent on radiomics licensed to Radiomics (Oncoradiomics SA). J. Guiot confirms that none of the above entities or funding was involved in the preparation of this work. A. Denis and P. Tsiri have no conflicts of interest to declare.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
