Hydric and Thermal Traits of Northern Australian Geckos: Water Loss Is Not Explained by Aridity
- PMID: 40529329
- PMCID: PMC12171639
- DOI: 10.1002/ece3.71585
Hydric and Thermal Traits of Northern Australian Geckos: Water Loss Is Not Explained by Aridity
Abstract
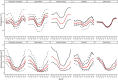
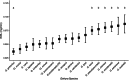
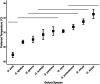
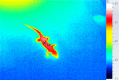
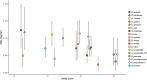
Behavioural and physiological adaptations are important for maintaining stable hydric states and viable body temperatures under challenging conditions experienced in variable terrestrial environments. For example, reptiles from arid locations tend to have lower rates of evaporative water loss (EWL). Here we test the prediction that geckos adapt their physiology to match the local environment to reduce hydric and thermal stress. Specifically, we compared EWL and preferred temperature among closely related species living under a range of climatic conditions. EWL rates were measured using a flow-through system in 18 species in the genus Gehyra collected from 11 locations across a strong gradient in aridity in tropical northern Australia during the dry season (Austral winter), and preferred temperatures were measured for nine of these species. Rates of EWL did not differ significantly among most species except between those with the highest and lowest rates. There was no association between EWL and the aridity of the locations where geckos were captured, and microhabitat conditions (temperature and humidity in rock crevices, used as retreats) did not explain this lack of association. Thermal preferences differed among species, with G. koira selecting significantly cooler temperatures than all other species. Gehyra moritzi, from the most arid and hottest location (Kurundi Station), had the highest preferred body temperature, overlapping only with two sympatric species (G. minuta and G. purpurascens). Unlike some reptiles, there was no evidence Gehyra geckos specialise in their EWL to match the local climate despite the strong gradient in aridity across our sampling sites. Nocturnal activity or seasonal plasticity in EWL may explain the lack of association between physiological traits of these species and the broad climatic conditions in the places they live.
Keywords: Gehyra; aridity; climate; evaporative water loss; phylogeny; thermal preference.
© 2025 The Author(s). Ecology and Evolution published by British Ecological Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Bates, D. , Mächler M., Bolker B., and Walker S.. 2015. “Fitting Linear Mixed‐Effects Models Using lme4.” Journal of Statistical Software 67: 1–48. 10.48550/arXiv.1406.5823. - DOI
-
- Belasen, A. , Brock K., Li B., et al. 2017. “Fine With Heat, Problems With Water: Microclimate Alters Water Loss in a Thermally Adapted Insular Lizard.” Oikos 126: 447–457. 10.5061/dryad.kp140. - DOI
-
- Blamires, S. J. , and Christian K. A.. 1999. “Seasonal Water Loss of the Lizard Lophognathus Temporalis in the Wet‐Dry Tropics of Northern Australia.” Amphibia‐Reptilia 20: 211–215. 10.1163/156853899X00213. - DOI
Associated data
LinkOut - more resources
Full Text Sources

